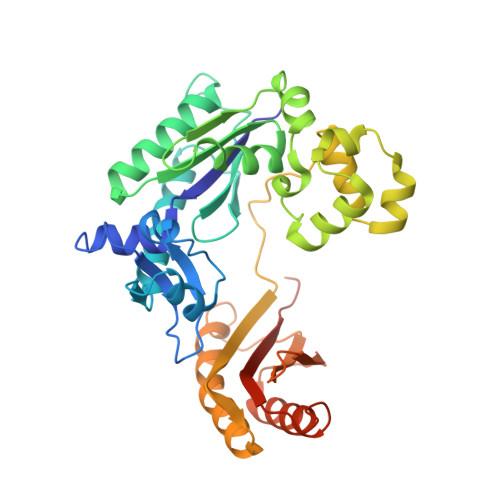
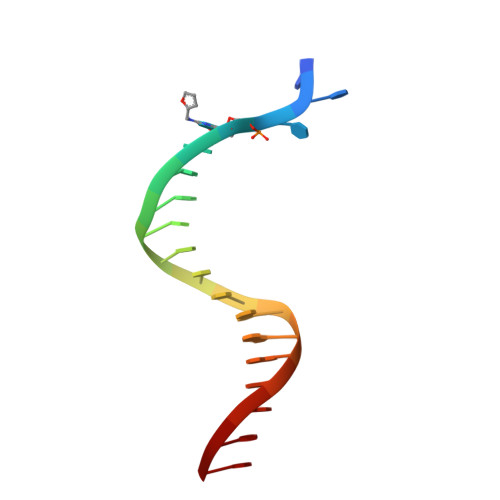
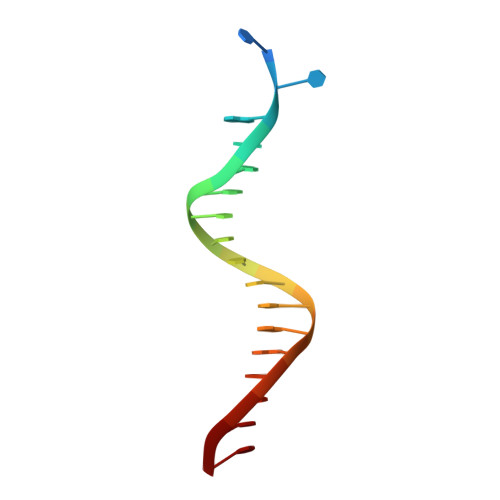
Unique structural features in DNA polymerase IV enable efficient bypass of the N2 adduct induced by the nitrofurazone antibiotic
Kottur, J., Sharma, A., Gore, K.R., Narayanan, N., Samanta, B., Pradeepkumar, P.I., Nair, D.T.(2015) Structure 23: 56-67
- PubMed: 25497730
- DOI: https://doi.org/10.1016/j.str.2014.10.019
- Primary Citation of Related Structures:
4Q43, 4Q44, 4Q45 - PubMed Abstract:
The reduction in the efficacy of therapeutic antibiotics represents a global problem of increasing intensity and concern. Nitrofuran antibiotics act primarily through the formation of covalent adducts at the N(2) atom of the deoxyguanosine nucleotide in genomic DNA. These adducts inhibit replicative DNA polymerases (dPols), leading to the death of the prokaryote. N(2)-furfuryl-deoxyguanosine (fdG) represents a stable structural analog of the nitrofuran-induced adducts. Unlike other known dPols, DNA polymerase IV (PolIV) from E. coli can bypass the fdG adduct accurately with high catalytic efficiency. This property of PolIV is central to its role in reducing the sensitivity of E. coli toward nitrofuran antibiotics such as nitrofurazone (NFZ). We present the mechanism used by PolIV to bypass NFZ-induced adducts and thus improve viability of E. coli in the presence of NFZ. Our results can be used to develop specific inhibitors of PolIV that may potentiate the activity of nitrofuran antibiotics.
- National Centre for Biological Sciences (NCBS-TIFR), GKVK Campus, Bellary Road, Bangalore 560065, India; Manipal University, Manipal.edu, Madhav Nagar, Manipal 576104, India.
Organizational Affiliation: