Alpha/beta hydrolase fold protein from Chitinophaga pinensis.
Osipiuk, J., Tesar, C., Clancy, S., Joachimiak, A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Alpha/beta hydrolase fold protein | 283 | Chitinophaga pinensis DSM 2588 | Mutation(s): 0 Gene Names: Cpin_2213 | 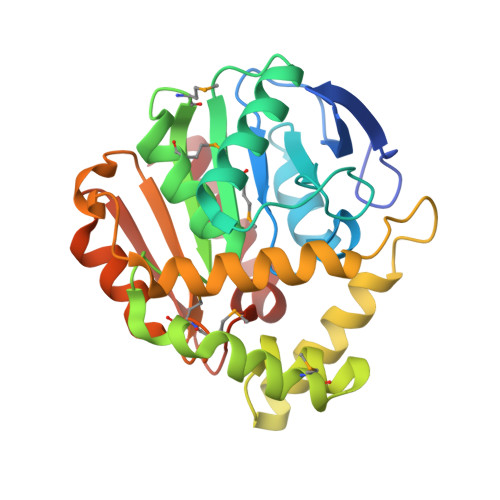 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CL Query on CL | B [auth A] C [auth A] D [auth A] E [auth A] F [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 110.38 | α = 90 |
| b = 110.38 | β = 90 |
| c = 59.184 | γ = 120 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| SBC-Collect | data collection |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| SHELXD | phasing |
| MLPHARE | phasing |
| DM | phasing |
| SOLVE | phasing |
| RESOLVE | phasing |
| HKL-3000 | phasing |