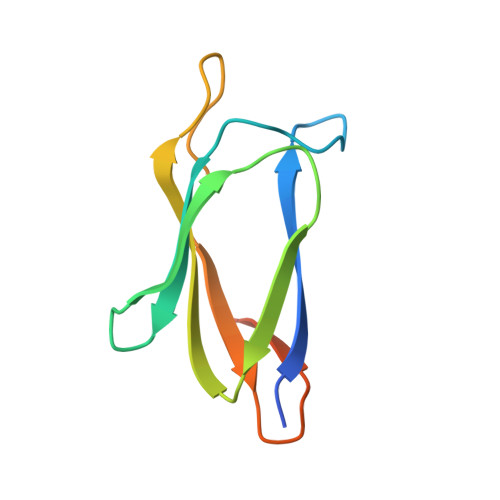
Structural Analysis and Optimization of the Covalent Association between SpyCatcher and a Peptide Tag.
Li, L., Fierer, J.O., Rapoport, T.A., Howarth, M.(2014) J Mol Biology 426: 309-317
- PubMed: 24161952
- DOI: https://doi.org/10.1016/j.jmb.2013.10.021
- Primary Citation of Related Structures:
4MLI, 4MLS - PubMed Abstract:
Peptide tagging is a key strategy for observing and isolating proteins. However, the interactions of proteins with peptides are nearly all rapidly reversible. Proteins tagged with the peptide SpyTag form an irreversible covalent bond to the SpyCatcher protein via a spontaneous isopeptide linkage, thereby offering a genetically encoded way to create peptide interactions that resist force and harsh conditions. Here, we determined the crystal structure of the reconstituted covalent complex of SpyTag and SpyCatcher at 2.1Å resolution. The structure showed the expected reformation of the β-sandwich domain seen in the parental streptococcal adhesin, but flanking sequences at both N- and C-termini of SpyCatcher were disordered. In addition, only 10 out of 13 amino acids of the SpyTag peptide were observed to interact with SpyCatcher, pointing to specific contacts important for rapid split protein reconstitution. Based on these structural insights, we expressed a range of SpyCatcher variants and identified a minimized SpyCatcher, 32 residues shorter, that maintained rapid reaction with SpyTag. Together, these results give insight into split protein β-strand complementation and enhance a distinct approach to ultrastable molecular interaction.
- Howard Hughes Medical Institute and Department of Cell Biology, Harvard Medical School, 240 Longwood Avenue, Boston, MA 02115, USA.
Organizational Affiliation: