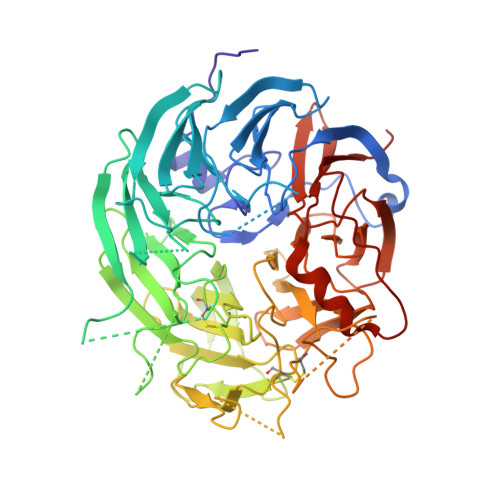
Crystal structure of L-sorbose dehydrogenase, a pyrroloquinoline quinone-dependent enzyme with homodimeric assembly, from Ketogulonicigenium vulgare
Han, X., Xiong, X., Jiang, D., Chen, S., Huang, E., Zhang, W., Liu, X.(2014) Biotechnol Lett 36: 1001-1008
- PubMed: 24557074
- DOI: https://doi.org/10.1007/s10529-013-1446-5
- Primary Citation of Related Structures:
4MH1 - PubMed Abstract:
The crystal structure of the L-sorbose dehydrogenase (SDH) from Ketogulonicigenium vulgare Y25 has been determined at 2.7 Å resolution using the molecular replacement method. The overall structure of SDH is similar to that of other quinoprotein dehydrogenases; consisting of an eight bladed β-propeller PQQ domain and protrusion loops. We identified a stable homodimer in crystal and demonstrated its existence in solution by sedimentation velocity measurement. By biochemical characterization of the SDH in vitro, using L-sorbose as substrate and cytochrome c551 as electron acceptor, we revealed cytochrome c551 acting as physiological primary electron acceptor for SDH.
- State Key Laboratory of Medicinal Chemical Biology, College of Life Sciences, Nankai University, Tianjin, 300071, China, hxdon@126.com.
Organizational Affiliation: