Structural insights into CNG-repetitive RNAs associated with human Trinucleotide Repeat Expansion Diseases (TREDs)
Tamjar, J., Katorcha, E., Cabo, A., Delgado, S., Popov, A.N., Malinina, L.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| RNA silencing suppressor p19 | A, C [auth D] | 135 | Tomato bushy stunt virus | Mutation(s): 2 Gene Names: ORF4 | 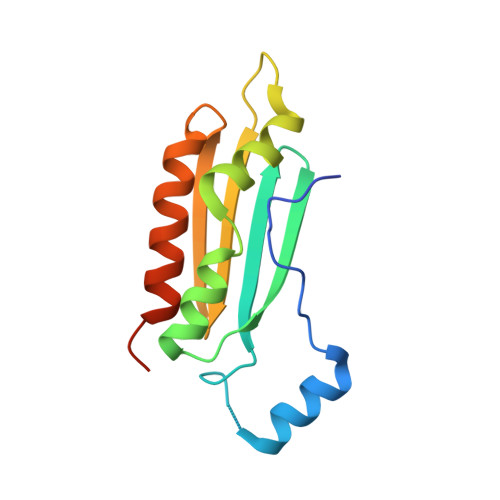 |
UniProt | |||||
Find proteins for P69517 (Tomato bushy stunt virus (strain Ja9)) Explore P69517 Go to UniProtKB: P69517 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P69517 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| 5'-R(P*GP*GP*CP*GP*GP*CP*GP*GP*CP*GP*GP*CP*GP*GP*CP*GP*GP*CP*C)-3' | B, D [auth E] | 19 | N/A | 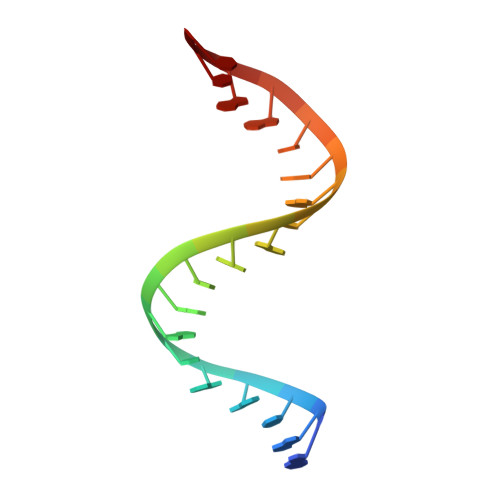 | |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | E [auth A] F [auth A] G [auth A] H [auth D] I [auth D] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 90.926 | α = 90 |
| b = 90.926 | β = 90 |
| c = 147.791 | γ = 120 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| ADSC | data collection |
| HKL-2000 | data reduction |
| REFMAC | phasing |