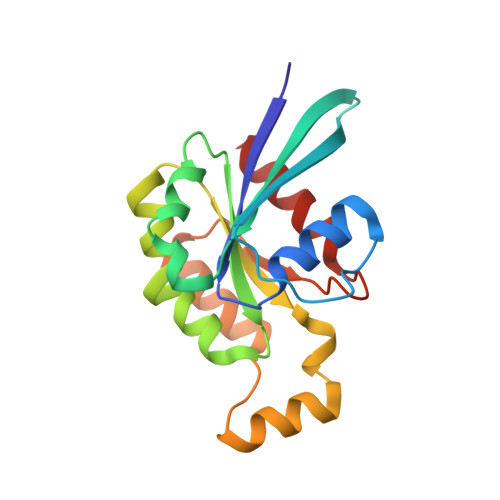
Mechanism of IRSp53 inhibition and combinatorial activation by Cdc42 and downstream effectors.
Kast, D.J., Yang, C., Disanza, A., Boczkowska, M., Madasu, Y., Scita, G., Svitkina, T., Dominguez, R.(2014) Nat Struct Mol Biol 21: 413-422
- PubMed: 24584464
- DOI: https://doi.org/10.1038/nsmb.2781
- Primary Citation of Related Structures:
4JS0 - PubMed Abstract:
The Rho family GTPase effector IRSp53 has essential roles in filopodia formation and neuronal development, but its regulatory mechanism is poorly understood. IRSp53 contains a membrane-binding BAR domain followed by an unconventional CRIB motif that overlaps with a proline-rich region (CRIB-PR) and an SH3 domain that recruits actin cytoskeleton effectors. Using a fluorescence reporter assay, we show that human IRSp53 adopts a closed inactive conformation that opens synergistically with the binding of human Cdc42 to the CRIB-PR and effector proteins, such as the tumor-promoting factor Eps8, to the SH3 domain. The crystal structure of Cdc42 bound to the CRIB-PR reveals a new mode of effector binding to Rho family GTPases. Structure-inspired mutations disrupt autoinhibition and Cdc42 binding in vitro and decouple Cdc42- and IRSp53-dependent filopodia formation in cells. The data support a combinatorial mechanism of IRSp53 activation.
- Department of Physiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Organizational Affiliation: