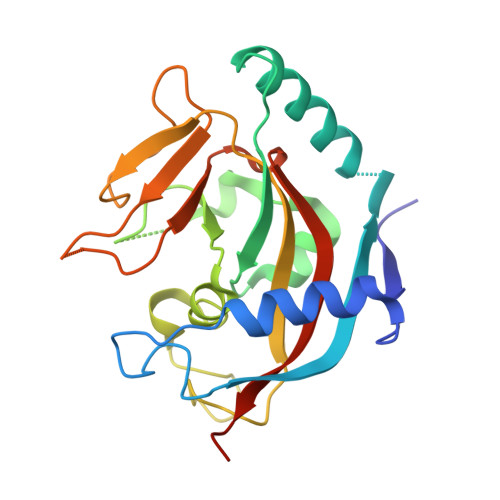
Novel binding mode of a potent and selective tankyrase inhibitor.
Gunaydin, H., Gu, Y., Huang, X.(2012) PLoS One 7: e33740-e33740
- PubMed: 22438990
- DOI: https://doi.org/10.1371/journal.pone.0033740
- Primary Citation of Related Structures:
4DVI - PubMed Abstract:
Tankyrases (TNKS1 and TNKS2) are key regulators of cellular processes such as telomere pathway and Wnt signaling. IWRs (inhibitors of Wnt response) have recently been identified as potent and selective inhibitors of tankyrases. However, it is not clear how these IWRs interact with tankyrases. Here we report the crystal structure of the catalytic domain of human TNKS1 in complex with IWR2, which reveals a novel binding site for tankyrase inhibitors. The TNKS1/IWR2 complex provides a molecular basis for their strong and specific interactions and suggests clues for further development of tankyrase inhibitors.
- Department of Molecular Structure, Amgen, Cambridge, Massachusetts, United States of America.
Organizational Affiliation: