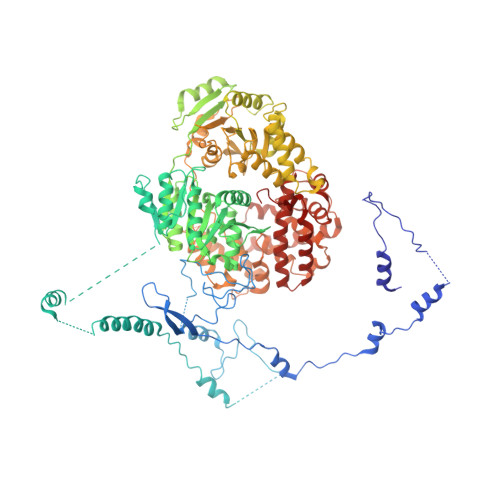
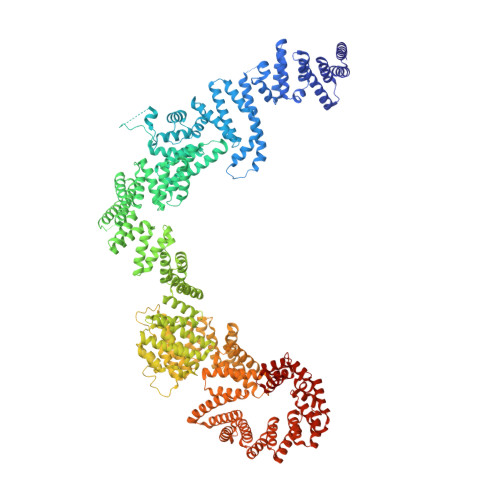
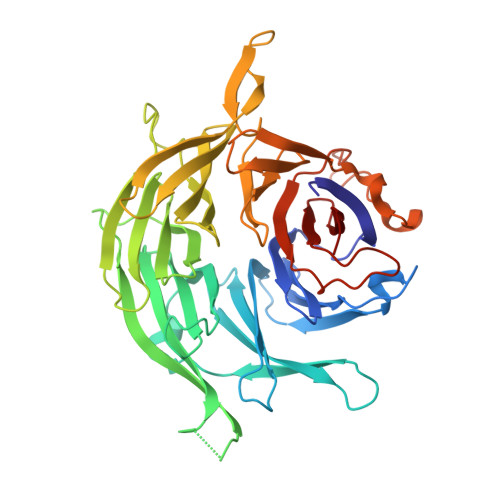
The Yeast Ski Complex: Crystal Structure and RNA Channeling to the Exosome Complex.
Halbach, F., Reichelt, P., Rode, M., Conti, E.(2013) Cell 154: 814
- PubMed: 23953113
- DOI: https://doi.org/10.1016/j.cell.2013.07.017
- Primary Citation of Related Structures:
4BUJ - PubMed Abstract:
The Ski complex is a conserved multiprotein assembly required for the cytoplasmic functions of the exosome, including RNA turnover, surveillance, and interference. Ski2, Ski3, and Ski8 assemble in a tetramer with 1:1:2 stoichiometry. The crystal structure of an S. cerevisiae 370 kDa core complex shows that Ski3 forms an array of 33 TPR motifs organized in N-terminal and C-terminal arms. The C-terminal arm of Ski3 and the two Ski8 subunits position the helicase core of Ski2 centrally within the complex, enhancing RNA binding. The Ski3 N-terminal arm and the Ski2 insertion domain allosterically modulate the ATPase and helicase activities of the complex. Biochemical data suggest that the Ski complex can thread RNAs directly to the exosome, coupling the helicase and the exoribonuclease through a continuous RNA channel. Finally, we identify a Ski8-binding motif common to Ski3 and Spo11, rationalizing the moonlighting properties of Ski8 in mRNA decay and meiosis.
- Department of Structural Cell Biology, Max Planck Institute of Biochemistry, Am Klopferspitz 18, 82152 Martinsried/Munich, Germany.
Organizational Affiliation: