Insights Into the Structural Determinants Required for High- Affinity Binding of Chiral Cyclopropane-Containing Ligands to Alpha4Beta2-Nicotinic Acetylcholine Receptors: An Integrated Approach to Behaviorally Active Nicotinic Ligands.
Zhang, H., Eaton, J.B., Yu, L., Nys, M., Mazzolari, A., Van Elk, R., Smit, A.B., Alexandrov, V., Hanania, T., Sabath, E., Fedolak, A., Brunner, D., Lukas, R.J., Vistoli, G., Ulens, C., Kozikowski, A.P.(2012) J Med Chem 55: 8028
- PubMed: 22928944
- DOI: https://doi.org/10.1021/jm3008739
- Primary Citation of Related Structures:
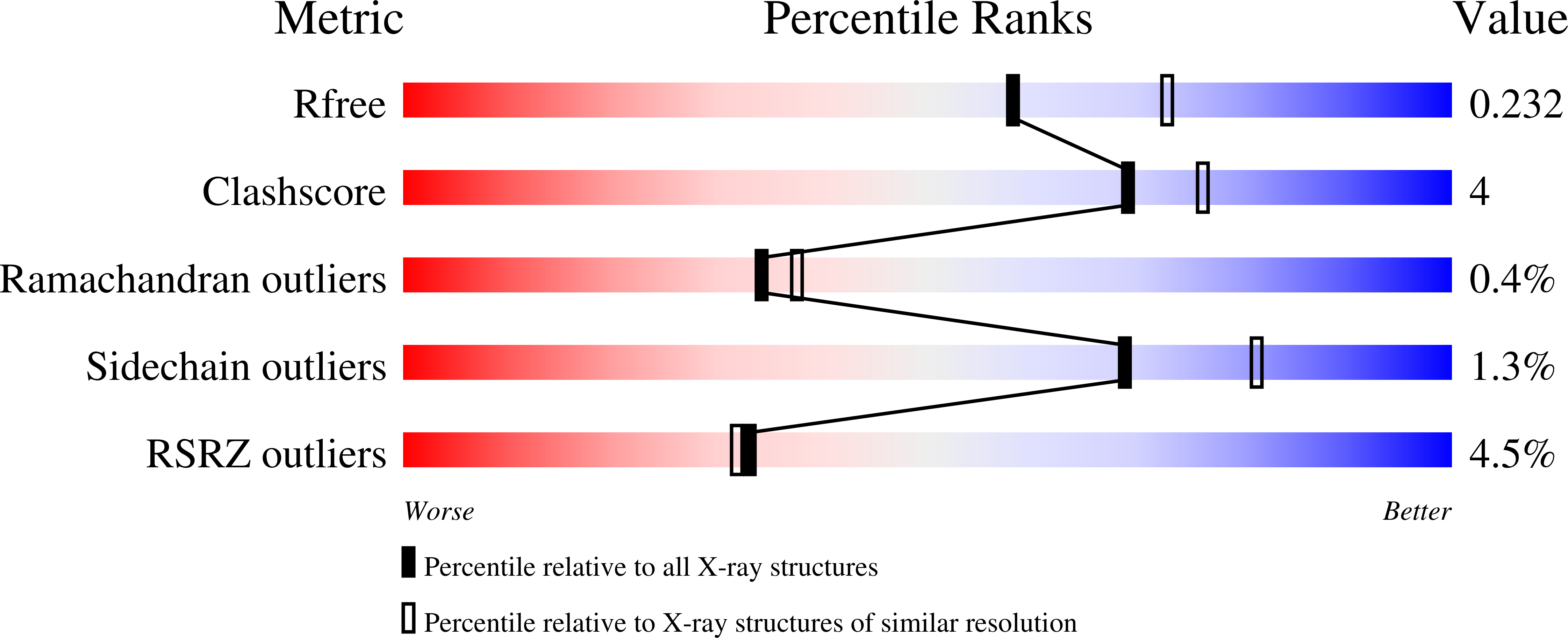
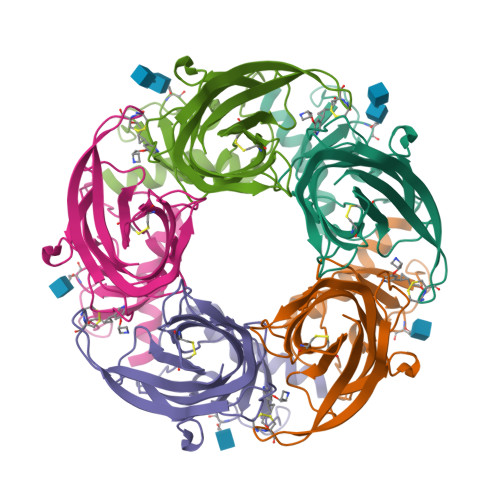
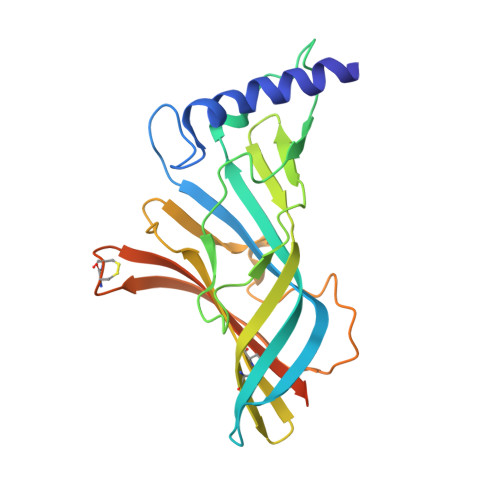
4B5D - PubMed Abstract:
Structure-based drug design can potentially accelerate the development of new therapeutics. In this study, a cocrystal structure of the acetylcholine binding protein (AChBP) from Capitella teleta (Ct) in complex with a cyclopropane-containing selective α4β2-nicotinic acetylcholine receptor (nAChR) partial agonist (compound 5) was acquired. The structural determinants required for ligand binding obtained from this AChBP X-ray structure were used to refine a previous model of the human α4β2-nAChR, thus possibly providing a better understanding of the structure of the human receptor. To validate the potential application of the structure of the Ct-AChBP in the engineering of new α4β2-nAChR ligands, homology modeling methods, combined with in silico ADME calculations, were used to design analogues of compound 5. The most promising compound, 12, exhibited an improved metabolic stability in comparison to the parent compound 5 while retaining favorable pharmacological parameters together with appropriate behavioral end points in the rodent studies.
Organizational Affiliation:
Drug Discovery Program, Department of Medicinal Chemistry and Pharmacognosy, University of Illinois at Chicago, 833 South Wood Street, Chicago, Illinois 60612, USA.