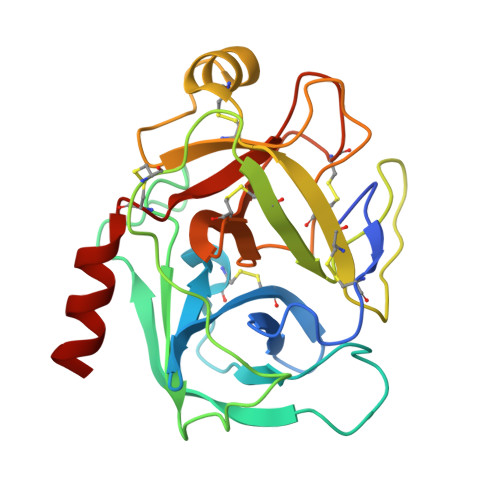
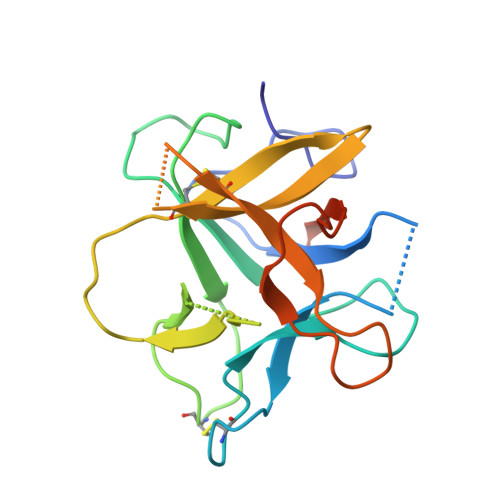
Structural Basis for Dual Inhibitory Role of Tamarind Kunitz Inhibitor (Tki) Against Factor Xa and Trypsin.
Patil, D.N., Chaudhary, A., Sharma, A.K., Tomar, S., Kumar, P.(2012) FEBS J 279: 4547
- PubMed: 23094997
- DOI: https://doi.org/10.1111/febs.12042
- Primary Citation of Related Structures:
4AN6, 4AN7 - PubMed Abstract:
A Kunitz type dual inhibitor (TKI) of factor Xa (FXa) and trypsin was found in tamarind. It also shows prolongation of blood coagulation time. The deduced 185 amino acid sequence of TKI by cDNA cloning and sequence analysis revealed that it belongs to the Kunitz type soybean trypsin inhibitor (STI) family; however, it has a distorted Kunitz signature sequence due to insertion of Asn15 in the motif. TKI exhibited a competitive inhibitory activity against both FXa (K(i) = 220 nm) and porcine pancreatic trypsin (K(i) = 3.2 nm). The crystal structure of TKI shows a β-trefoil fold similar to Kunitz STI inhibitors; however, a distinct mobile reactive site, an inserted residue and loop β7β8 make it distinct from classical Kunitz inhibitors. The crystal structure of TKI-trypsin and a 3D model of TKI-FXa complex revealed that the distinct reactive site loop probably plays a role in dual inhibition. The reactive site of TKI interacts with an active site and two exosites (36 loop and autolysis loop) of FXa. Apart from Arg66 (P1), Arg64 (P3) is one of the most important residues responsible for the specificity of TKI towards FXa. Along with the reactive site loop (β4β5), loops β1 and β7β8 also interact with FXa and could further confer selectivity for FXa. We also present the role of inserted Asn15 in the stabilization of complexes. To the best of our knowledge, this is the first structure of FXa inhibitor belonging to the Kunitz type inhibitor family and its unique structural and sequence features make TKI a novel potent inhibitor. The complete nucleotide of TKI was deposited in the NCBI gene databank with accession no. HQ385502. The atomic coordinates and structure factor files for the structure of TKI and TKI:PPT complex have been deposited in the Protein Data Bank with accession numbers 4AN6 and 4AN7, respectively TKI and TKI bind by x-ray crystallography (View interaction) TKI and PPT bind by x-ray crystallography (View interaction).
- Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand 247667, India.
Organizational Affiliation: