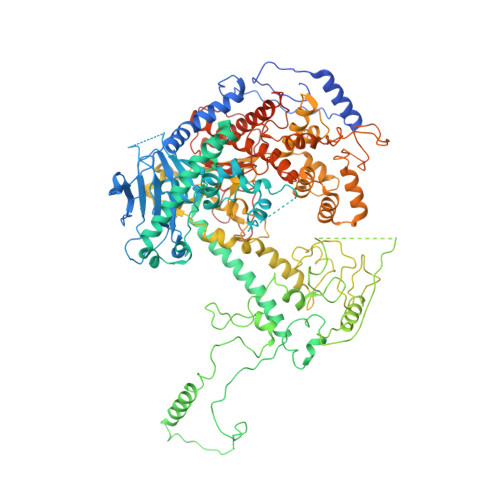
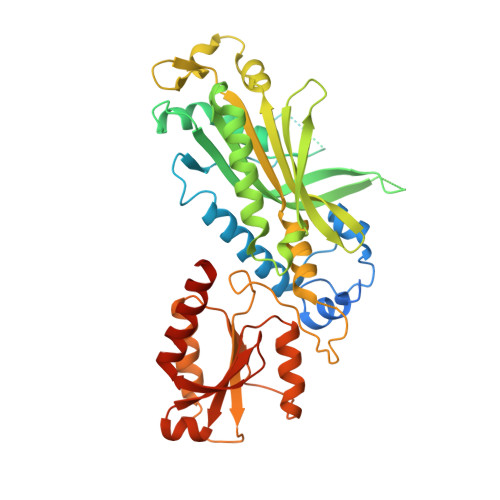
Structural basis for processivity and antiviral drug toxicity in human mitochondrial DNA replicase
Szymanski, M.R., Kuznestov, V.B., Shumate, C.K., Meng, Q., Lee, Y.-S., Patel, G., Patel, S.S., Yin, Y.W.(2015) EMBO J 34: 1959
- PubMed: 26056153
- DOI: https://doi.org/10.15252/embj.201591520
- Primary Citation of Related Structures:
4ZTU, 4ZTZ - PubMed Abstract:
The human DNA polymerase gamma (Pol γ) is responsible for DNA replication in mitochondria. Pol γ is particularly susceptible to inhibition by dideoxynucleoside-based inhibitors designed to fight viral infection. Here, we report crystal structures of the replicating Pol γ-DNA complex bound to either substrate or zalcitabine, an inhibitor used for HIV reverse transcriptase. The structures reveal that zalcitabine binds to the Pol γ active site almost identically to the substrate dCTP, providing a structural basis for Pol γ-mediated drug toxicity. When compared to the apo form, Pol γ undergoes intra- and inter-subunit conformational changes upon formation of the ternary complex with primer/template DNA and substrate. We also find that the accessory subunit Pol γB, which lacks intrinsic enzymatic activity and does not contact the primer/template DNA directly, serves as an allosteric regulator of holoenzyme activities. The structures presented here suggest a mechanism for processivity of the holoenzyme and provide a model for understanding the deleterious effects of Pol γ mutations in human disease. Crystal structures of the mitochondrial DNA polymerase, Pol γ, in complex with substrate or antiviral inhibitor zalcitabine provide a basis for understanding Pol γ-mediated drug toxicity.
- Department of Pharmacology and Toxicology, University of Texas Medical Branch, Galveston, TX, USA Sealy Center for Structural Biology, University of Texas Medical Branch, Galveston, TX, USA.
Organizational Affiliation: