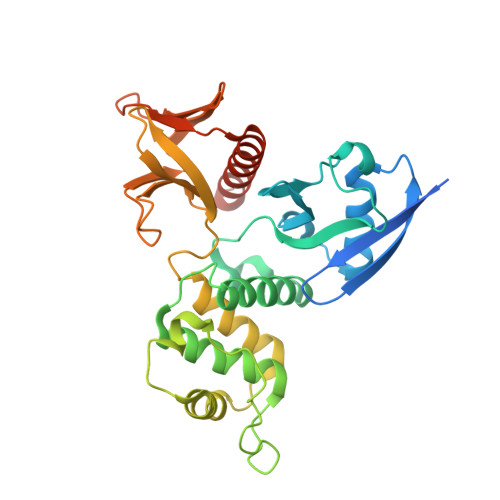
Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway
Li, Y., Zhou, H., Li, F., Chan, S.W., Lin, Z., Wei, Z., Yang, Z., Guo, F., Lim, C.J., Xing, W., Shen, Y., Hong, W., Long, J., Zhang, M.(2015) Cell Res 25: 801-817
- PubMed: 26045165
- DOI: https://doi.org/10.1038/cr.2015.69
- Primary Citation of Related Structures:
4ZRI, 4ZRJ, 4ZRK - PubMed Abstract:
The tumor suppressor Merlin/NF2 functions upstream of the core Hippo pathway kinases Lats1/2 and Mst1/2, as well as the nuclear E3 ubiquitin ligase CRL4(DCAF1). Numerous mutations of Merlin have been identified in Neurofibromatosis type 2 and other cancer patients. Despite more than two decades of research, the upstream regulator of Merlin in the Hippo pathway remains unknown. Here we show by high-resolution crystal structures that the Lats1/2-binding site on the Merlin FERM domain is physically blocked by Merlin's auto-inhibitory tail. Angiomotin binding releases the auto-inhibition and promotes Merlin's binding to Lats1/2. Phosphorylation of Ser518 outside the Merlin's auto-inhibitory tail does not obviously alter Merlin's conformation, but instead prevents angiomotin from binding and thus inhibits Hippo pathway kinase activation. Cancer-causing mutations clustered in the angiomotin-binding domain impair angiomotin-mediated Merlin activation. Our findings reveal that angiomotin and Merlin respectively interface cortical actin filaments and core kinases in Hippo signaling, and allow construction of a complete Hippo signaling pathway.
- Division of Life Science, State Key Laboratory of Molecular Neuroscience, Hong Kong, China.
Organizational Affiliation: