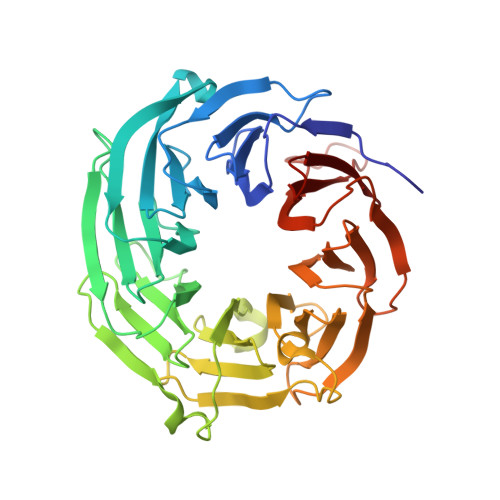
Co-translational capturing of nascent ribosomal proteins by their dedicated chaperones.
Pausch, P., Singh, U., Ahmed, Y.L., Pillet, B., Murat, G., Altegoer, F., Stier, G., Thoms, M., Hurt, E., Sinning, I., Bange, G., Kressler, D.(2015) Nat Commun 6: 7494-7494
- PubMed: 26112308
- DOI: https://doi.org/10.1038/ncomms8494
- Primary Citation of Related Structures:
4ZN4, 4ZOV, 4ZOX, 4ZOY, 4ZOZ - PubMed Abstract:
Exponentially growing yeast cells produce every minute >160,000 ribosomal proteins. Owing to their difficult physicochemical properties, the synthesis of assembly-competent ribosomal proteins represents a major challenge. Recent evidence highlights that dedicated chaperone proteins recognize the N-terminal regions of ribosomal proteins and promote their soluble expression and delivery to the assembly site. Here we explore the intuitive possibility that ribosomal proteins are captured by dedicated chaperones in a co-translational manner. Affinity purification of four chaperones (Rrb1, Syo1, Sqt1 and Yar1) selectively enriched the mRNAs encoding their specific ribosomal protein clients (Rpl3, Rpl5, Rpl10 and Rps3). X-ray crystallography reveals how the N-terminal, rRNA-binding residues of Rpl10 are shielded by Sqt1's WD-repeat β-propeller, providing mechanistic insight into the incorporation of Rpl10 into pre-60S subunits. Co-translational capturing of nascent ribosomal proteins by dedicated chaperones constitutes an elegant mechanism to prevent unspecific interactions and aggregation of ribosomal proteins on their road to incorporation.
- LOEWE Center for Synthetic Microbiology (SYNMIKRO) and Department of Chemistry, Philipps-University Marburg, Hans-Meerwein-Straße, Marburg D-35043, Germany.
Organizational Affiliation: