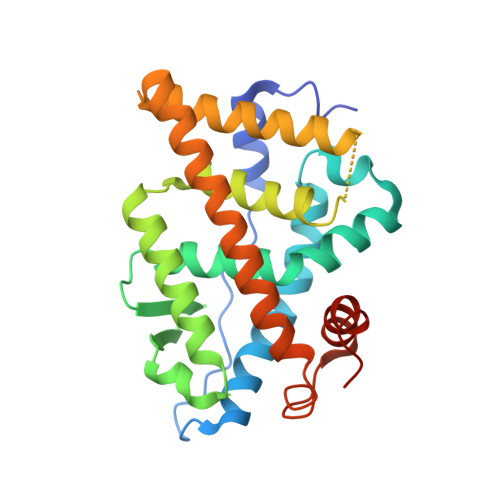
Development of selective estrogen receptor modulator (SERM)-like activity through an indirect mechanism of estrogen receptor antagonism: defining the binding mode of 7-oxabicyclo[2.2.1]hept-5-ene scaffold core ligands.
Zheng, Y., Zhu, M., Srinivasan, S., Nwachukwu, J.C., Cavett, V., Min, J., Carlson, K.E., Wang, P., Dong, C., Katzenellenbogen, J.A., Nettles, K.W., Zhou, H.B.(2012) ChemMedChem 7: 1094-1100
- PubMed: 22517684
- DOI: https://doi.org/10.1002/cmdc.201200048
- Primary Citation of Related Structures:
4ZN9 - PubMed Abstract:
Previously, we discovered estrogen receptor (ER) ligands with a novel three-dimensional oxabicyclo[2.2.1]heptene core scaffold and good ER binding affinity act as partial agonists via small alkyl ester substitutions on the bicyclic core that indirectly modulate the critical switch helix in the ER ligand binding domain, helix 12, by interactions with helix 11. This contrasts with the mechanism of action of tamoxifen, which directly pushes helix 12 out of the conformation required for gene activation. We now report that a much larger substitution can be tolerated at this position of the bicyclic core scaffold, namely a phenyl sulfonate group, which defines a novel binding epitope for the estrogen receptor. We prepared an array of 14 oxabicycloheptene sulfonates, varying the phenyl sulfonate group. As with the parent compound, 5,6-bis-(4-hydroxyphenyl)-7-oxabicyclo[2.2.1]hept-5-ene-2-sulfonic acid phenyl ester (OBHS), these compounds showed preferential affinity for ERα, and the disposition and size of the phenyl substituents were important determinants of the binding affinity and selectivity of these compounds, with those having ortho substituents giving the highest, and para substituents the lowest affinities for ERα. A few analogues exhibit ERα binding affinities that are comparable to or, in the case of the ortho-chloro analogue, higher than that of OBHS itself. In cell-based studies, we found several compounds with activity profiles comparable to tamoxifen, but acting entirely as indirect antagonists, allosterically interfering with recruitment of coactivator proteins to the receptor. Thus, the OBHS binding epitope represents a novel approach to the development of estrogen receptor antagonists via an indirect mechanism of antagonism.
- State Key Laboratory of Virology, Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education, School of Pharmaceutical Sciences, Wuhan University, 185 East Lake Road, Wuhan, 430071, PR China.
Organizational Affiliation: