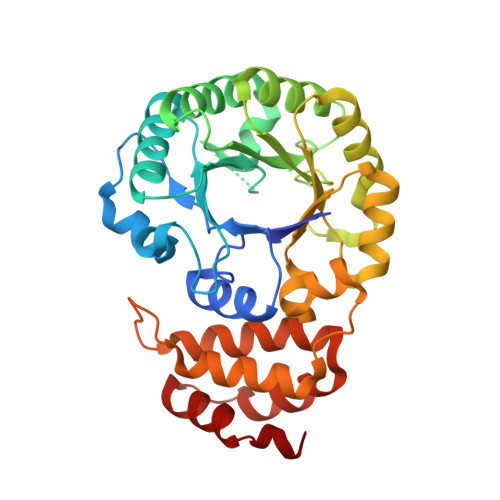
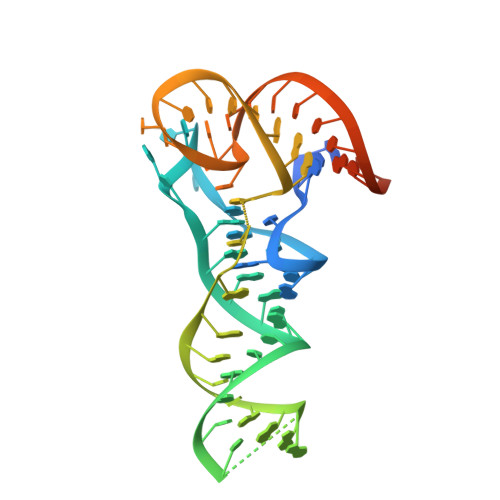
Major reorientation of tRNA substrates defines specificity of dihydrouridine synthases.
Byrne, R.T., Jenkins, H.T., Peters, D.T., Whelan, F., Stowell, J., Aziz, N., Kasatsky, P., Rodnina, M.V., Koonin, E.V., Konevega, A.L., Antson, A.A.(2015) Proc Natl Acad Sci U S A 112: 6033-6037
- PubMed: 25902496
- DOI: https://doi.org/10.1073/pnas.1500161112
- Primary Citation of Related Structures:
4BF9, 4BFA, 4YCO, 4YCP - PubMed Abstract:
The reduction of specific uridines to dihydrouridine is one of the most common modifications in tRNA. Increased levels of the dihydrouridine modification are associated with cancer. Dihydrouridine synthases (Dus) from different subfamilies selectively reduce distinct uridines, located at spatially unique positions of folded tRNA, into dihydrouridine. Because the catalytic center of all Dus enzymes is conserved, it is unclear how the same protein fold can be reprogrammed to ensure that nucleotides exposed at spatially distinct faces of tRNA can be accommodated in the same active site. We show that the Escherichia coli DusC is specific toward U16 of tRNA. Unexpectedly, crystal structures of DusC complexes with tRNA(Phe) and tRNA(Trp) show that Dus subfamilies that selectively modify U16 or U20 in tRNA adopt identical folds but bind their respective tRNA substrates in an almost reverse orientation that differs by a 160° rotation. The tRNA docking orientation appears to be guided by subfamily-specific clusters of amino acids ("binding signatures") together with differences in the shape of the positively charged tRNA-binding surfaces. tRNA orientations are further constrained by positional differences between the C-terminal "recognition" domains. The exquisite substrate specificity of Dus enzymes is therefore controlled by a relatively simple mechanism involving major reorientation of the whole tRNA molecule. Such reprogramming of the enzymatic specificity appears to be a unique evolutionary solution for altering tRNA recognition by the same protein fold.
- York Structural Biology Laboratory, Department of Chemistry, and.
Organizational Affiliation: