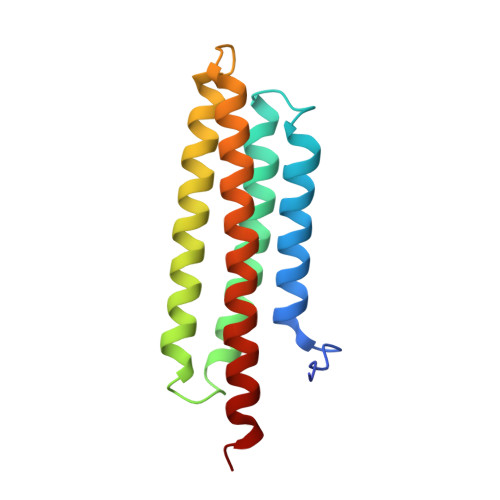
Structural Basis for the Interaction between Pyk2-FAT Domain and Leupaxin LD Repeats.
Vanarotti, M.S., Finkelstein, D.B., Guibao, C.D., Nourse, A., Miller, D.J., Zheng, J.J.(2016) Biochemistry 55: 1332-1345
- PubMed: 26866573
- DOI: https://doi.org/10.1021/acs.biochem.5b01274
- Primary Citation of Related Structures:
4XEF, 4XEK, 4XEV - PubMed Abstract:
Proline-rich tyrosine kinase 2 (Pyk2) is a nonreceptor tyrosine kinase and belongs to the focal adhesion kinase (FAK) family. Like FAK, the C-terminal focal adhesion-targeting (FAT) domain of Pyk2 binds to paxillin, a scaffold protein in focal adhesions; however, the interaction between the FAT domain of Pyk2 and paxillin is dynamic and unstable. Leupaxin is another member in the paxillin family and was suggested to be the native binding partner of Pyk2; Pyk2 gene expression is strongly correlated with that of leupaxin in many tissues including primary breast cancer. Here, we report that leupaxin interacts with Pyk2-FAT. Leupaxin has four leucine-aspartate (LD) motifs. The first and third LD motifs of leupaxin preferably target the two LD-binding sites on the Pyk2-FAT domain, respectively. Moreover, the full-length leupaxin binds to Pyk2-FAT as a stable one-to-one complex. Together, we propose that there is an underlying selectivity between leupaxin and paxillin for Pyk2, which may influence the differing behavior of the two proteins at focal adhesion sites.
- Stein Eye Institute, Department of Ophthalmology, David Geffen School of Medicine at UCLA , Los Angeles, California 90095, United States.
Organizational Affiliation: