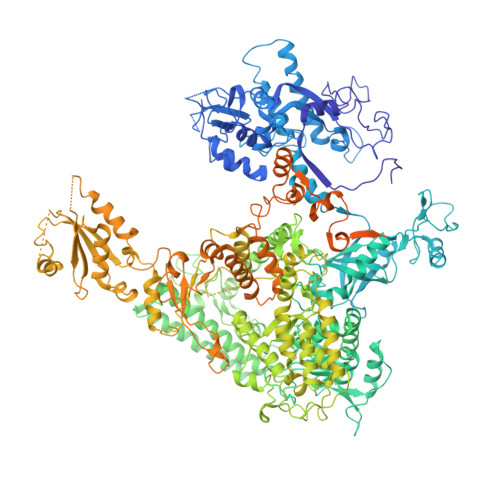
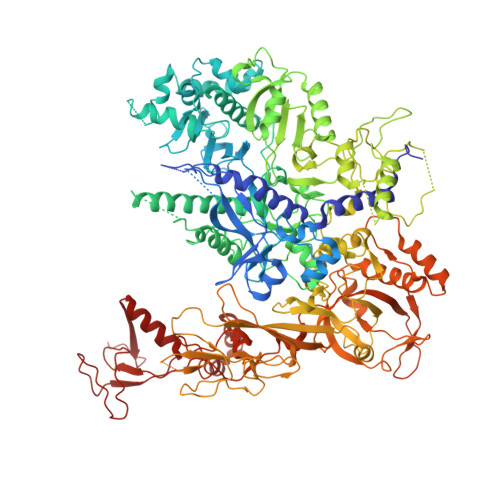
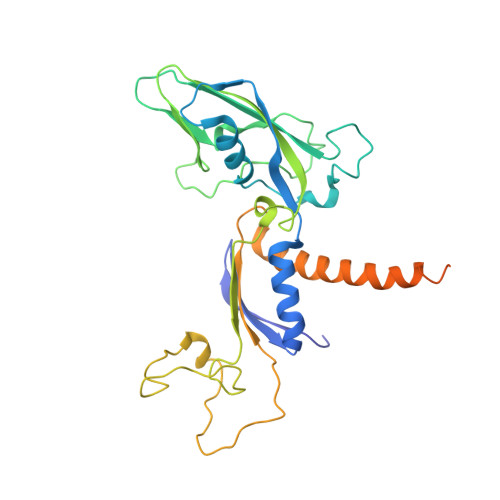
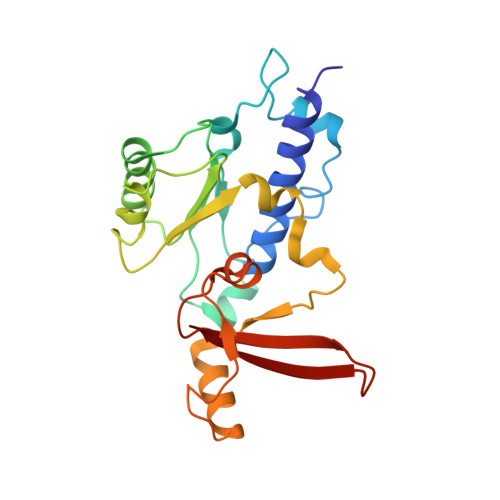
Mechanism of RNA polymerase II bypass of oxidative cyclopurine DNA lesions.
Walmacq, C., Wang, L., Chong, J., Scibelli, K., Lubkowska, L., Gnatt, A., Brooks, P.J., Wang, D., Kashlev, M.(2015) Proc Natl Acad Sci U S A 112: E410-E419
- PubMed: 25605892
- DOI: https://doi.org/10.1073/pnas.1415186112
- Primary Citation of Related Structures:
4X67, 4X6A - PubMed Abstract:
In human cells, the oxidative DNA lesion 8,5'-cyclo-2'-deoxyadenosine (CydA) induces prolonged stalling of RNA polymerase II (Pol II) followed by transcriptional bypass, generating both error-free and mutant transcripts with AMP misincorporated immediately downstream from the lesion. Here, we present biochemical and crystallographic evidence for the mechanism of CydA recognition. Pol II stalling results from impaired loading of the template base (5') next to CydA into the active site, leading to preferential AMP misincorporation. Such predominant AMP insertion, which also occurs at an abasic site, is unaffected by the identity of the 5'-templating base, indicating that it derives from nontemplated synthesis according to an A rule known for DNA polymerases and recently identified for Pol II bypass of pyrimidine dimers. Subsequent to AMP misincorporation, Pol II encounters a major translocation block that is slowly overcome. Thus, the translocation block combined with the poor extension of the dA.rA mispair reduce transcriptional mutagenesis. Moreover, increasing the active-site flexibility by mutation in the trigger loop, which increases the ability of Pol II to accommodate the bulky lesion, and addition of transacting factor TFIIF facilitate CydA bypass. Thus, blocking lesion entry to the active site, translesion A rule synthesis, and translocation block are common features of transcription across different bulky DNA lesions.
- Center for Cancer Research, National Cancer Institute, Frederick, MD 21702;
Organizational Affiliation: