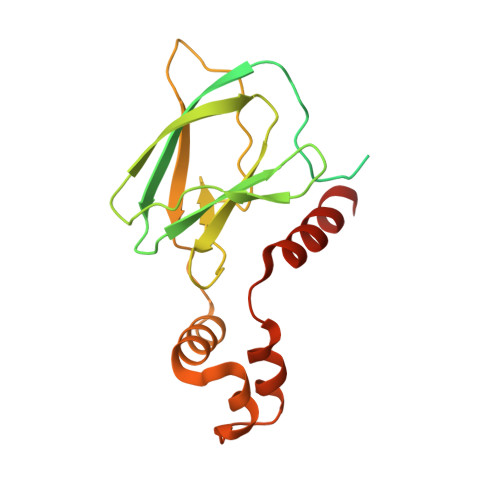
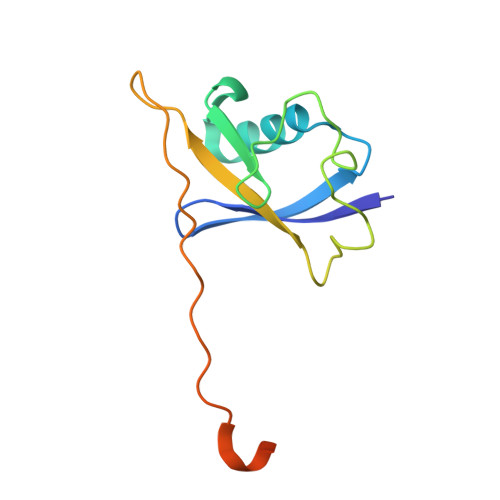
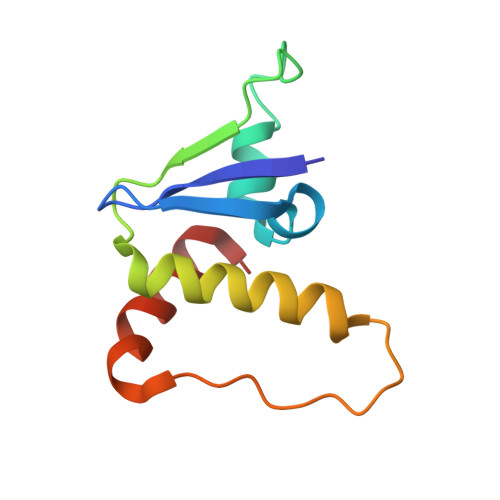
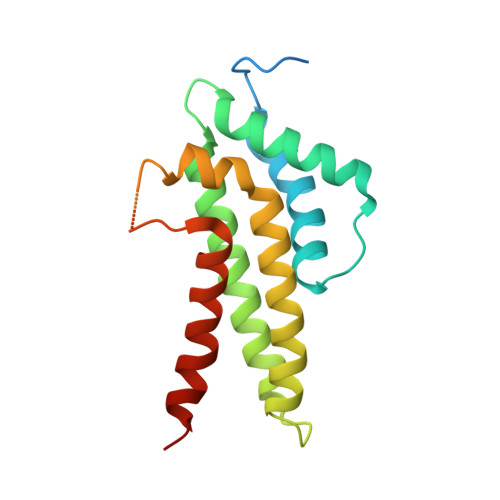
Insights into Cullin-RING E3 Ubiquitin Ligase Recruitment: Structure of the VHL-EloBC-Cul2 Complex.
Nguyen, H.C., Yang, H., Fribourgh, J.L., Wolfe, L.S., Xiong, Y.(2015) Structure 23: 441-449
- PubMed: 25661653
- DOI: https://doi.org/10.1016/j.str.2014.12.014
- Primary Citation of Related Structures:
4WQO - PubMed Abstract:
The von Hippel-Lindau tumor suppressor protein (VHL) recruits a Cullin 2 (Cul2) E3 ubiquitin ligase to downregulate HIF-1α, an essential transcription factor for the hypoxia response. Mutations in VHL lead to VHL disease and renal cell carcinomas. Inhibition of this pathway to upregulate erythropoietin production is a promising new therapy to treat ischemia and chronic anemia. Here, we report the crystal structure of VHL bound to a Cul2 N-terminal domain, Elongin B, and Elongin C (EloC). Cul2 interacts with both the VHL BC box and cullin box and a novel EloC site. Comparison with other cullin E3 ligase structures shows that there is a conserved, yet flexible, cullin recognition module and that cullin selectivity is influenced by distinct electrostatic interactions. Our structure provides a structural basis for the study of the pathogenesis of VHL disease and rationale for the design of novel compounds that may modulate cullin-substrate receptor interactions.
- Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06511, USA.
Organizational Affiliation: