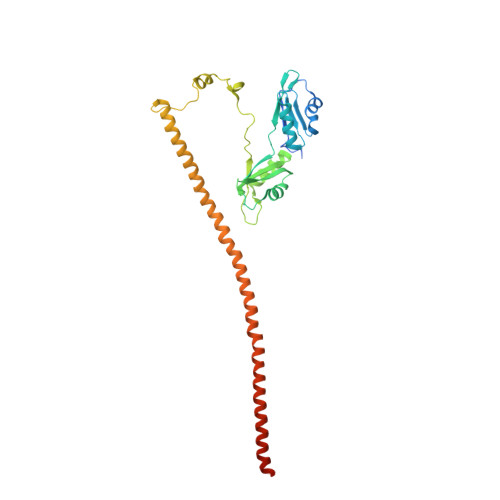
The structure of human SFPQ reveals a coiled-coil mediated polymer essential for functional aggregation in gene regulation.
Lee, M., Sadowska, A., Bekere, I., Ho, D., Gully, B.S., Lu, Y., Iyer, K.S., Trewhella, J., Fox, A.H., Bond, C.S.(2015) Nucleic Acids Res 43: 3826-3840
- PubMed: 25765647
- DOI: https://doi.org/10.1093/nar/gkv156
- Primary Citation of Related Structures:
4WII, 4WIJ, 4WIK - PubMed Abstract:
SFPQ, (a.k.a. PSF), is a human tumor suppressor protein that regulates many important functions in the cell nucleus including coordination of long non-coding RNA molecules into nuclear bodies. Here we describe the first crystal structures of Splicing Factor Proline and Glutamine Rich (SFPQ), revealing structural similarity to the related PSPC1/NONO heterodimer and a strikingly extended structure (over 265 Å long) formed by an unusual anti-parallel coiled-coil that results in an infinite linear polymer of SFPQ dimers within the crystals. Small-angle X-ray scattering and transmission electron microscopy experiments show that polymerization is reversible in solution and can be templated by DNA. We demonstrate that the ability to polymerize is essential for the cellular functions of SFPQ: disruptive mutation of the coiled-coil interaction motif results in SFPQ mislocalization, reduced formation of nuclear bodies, abrogated molecular interactions and deficient transcriptional regulation. The coiled-coil interaction motif thus provides a molecular explanation for the functional aggregation of SFPQ that directs its role in regulating many aspects of cellular nucleic acid metabolism.
- School of Chemistry and Biochemistry, University of Western Australia, 35 Stirling Highway, Crawley, Western Australia 6009, Australia.
Organizational Affiliation: