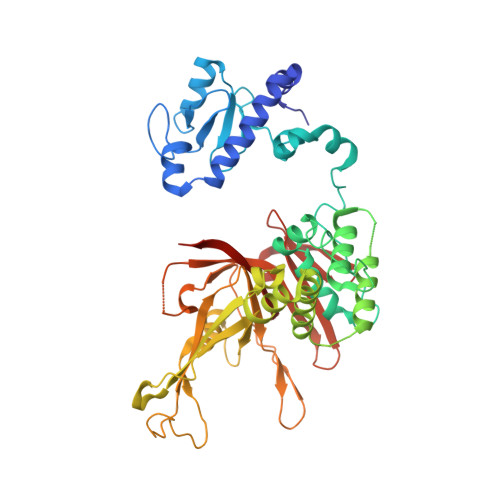
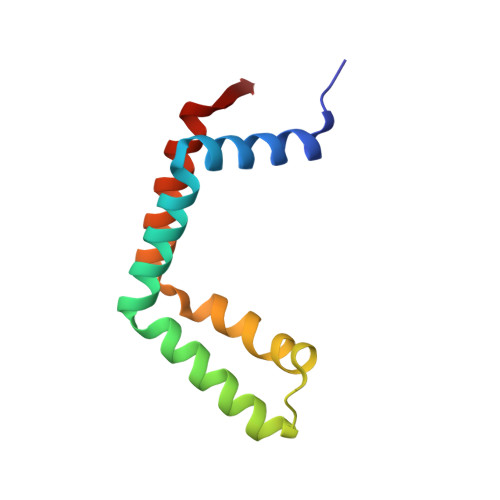
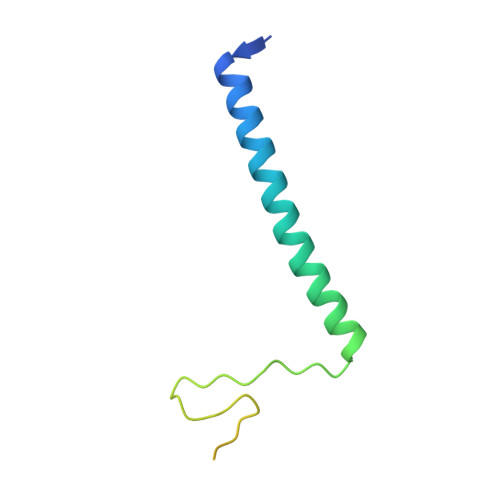
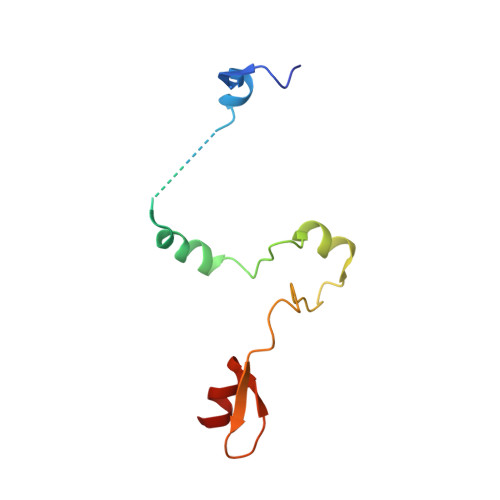
Uncovering the role of Sgf73 in maintaining SAGA deubiquitinating module structure and activity.
Yan, M., Wolberger, C.(2015) J Mol Biology 427: 1765-1778
- PubMed: 25526805
- DOI: https://doi.org/10.1016/j.jmb.2014.12.004
- Primary Citation of Related Structures:
4W4U - PubMed Abstract:
The SAGA (Spt-Ada-Gcn5 acetyltransferase) complex performs multiple functions in transcription activation including deubiquitinating histone H2B, which is mediated by a subcomplex called the deubiquitinating module (DUBm). The yeast DUBm comprises a catalytic subunit, Ubp8, and three additional subunits, Sgf11, Sus1 and Sgf73, all of which are required for DUBm activity. A portion of the non-globular Sgf73 subunit lies between the Ubp8 catalytic domain and the ZnF-UBP domain and has been proposed to contribute to deubiquitinating activity by maintaining the catalytic domain in an active conformation. We report structural and solution studies of the DUBm containing two different Sgf73 point mutations that disrupt deubiquitinating activity. We find that the Sgf73 mutations abrogate deubiquitinating activity by impacting the Ubp8 ubiquitin-binding fingers region and they have an unexpected effect on the overall folding and stability of the DUBm complex. Taken together, our data suggest a role for Sgf73 in maintaining both the organization and the ubiquitin-binding conformation of Ubp8, thereby contributing to overall DUBm activity.
- Department of Biophysics and Biophysical Chemistry, The Johns Hopkins University School of Medicine, 725 North Wolfe Street, Baltimore, MD 21205, USA.
Organizational Affiliation: