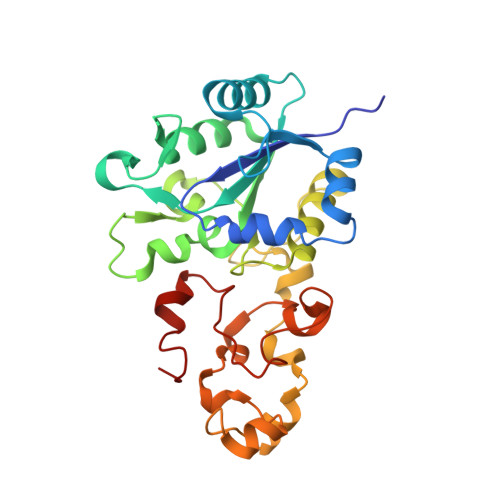
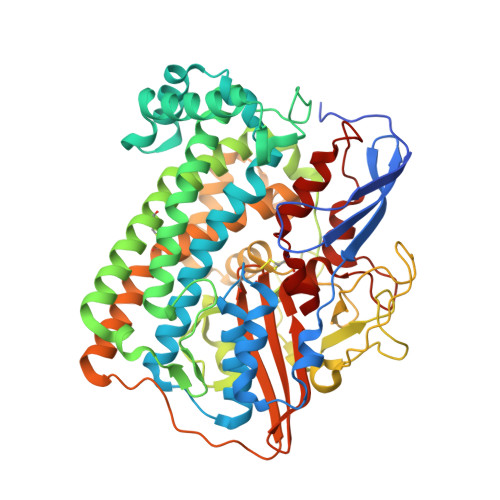
Crystallographic Studies of [Nife]-Hydrogenase Mutants: Towards Consensus Structures for the Elusive Unready Oxidized States.
Volbeda, A., Martin, L., Barbier, E., Gutierrez-Sanz, O., De Lacey, A.L., Liebgott, P., Dementin, S., Rousset, M., Fontecilla-Camps, J.C.(2015) J Biol Inorg Chem 20: 11
- PubMed: 25315838
- DOI: https://doi.org/10.1007/s00775-014-1203-9
- Primary Citation of Related Structures:
4UPE, 4UPV, 4UQL, 4UQP, 4URH - PubMed Abstract:
Catalytically inactive oxidized O2-sensitive [NiFe]-hydrogenases are characterized by a mixture of the paramagnetic Ni-A and Ni-B states. Upon O2 exposure, enzymes in a partially reduced state preferentially form the unready Ni-A state. Because partial O2 reduction should generate a peroxide intermediate, this species was previously assigned to the elongated Ni-Fe bridging electron density observed for preparations of [NiFe]-hydrogenases known to contain the Ni-A state. However, this proposition has been challenged based on the stability of this state to UV light exposure and the possibility of generating it anaerobically under either chemical or electrochemical oxidizing conditions. Consequently, we have considered alternative structures for the Ni-A species including oxidation of thiolate ligands to either sulfenate or sulfenic acid. Here, we report both new and revised [NiFe]-hydrogenases structures and conclude, taking into account corresponding characterizations by Fourier transform infrared spectroscopy (FTIR), that the Ni-A species contains oxidized cysteine and bridging hydroxide ligands instead of the peroxide ligand we proposed earlier. Our analysis was rendered difficult by the typical formation of mixtures of unready oxidized states that, furthermore, can be reduced by X-ray induced photoelectrons. The present study could be carried out thanks to the use of Desulfovibrio fructosovorans [NiFe]-hydrogenase mutants with special properties. In addition to the Ni-A state, crystallographic results are also reported for two diamagnetic unready states, allowing the proposal of a revised oxidized inactive Ni-SU model and a new structure characterized by a persulfide ion that is assigned to an Ni-'Sox' species.
- University Grenoble Alpes, IBS, 38044, Grenoble, France. anne.volbeda@ibs.fr.
Organizational Affiliation: