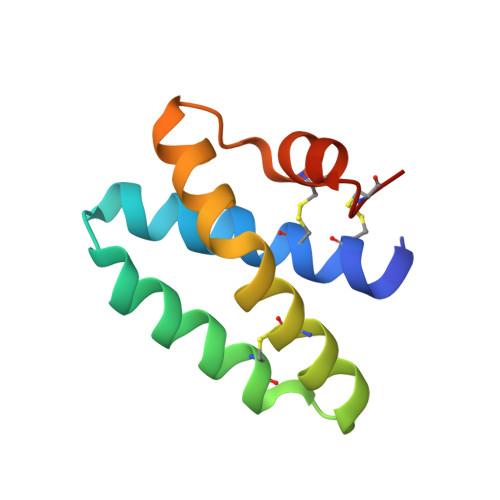
Structure of Human Saposin a at Lysosomal Ph.
Hill, C.H., Read, R.J., Deane, J.E.(2015) Acta Crystallogr D Biol Crystallogr 71: 895
- PubMed: 26144235
- DOI: https://doi.org/10.1107/S2053230X15008584
- Primary Citation of Related Structures:
4UEX - PubMed Abstract:
The saposins are essential cofactors for the normal lysosomal degradation of complex glycosphingolipids by acid hydrolase enzymes; defects in either saposin or hydrolase function lead to severe metabolic diseases. Saposin A (SapA) activates the enzyme β-galactocerebrosidase (GALC), which catalyzes the breakdown of β-D-galactocerebroside, the principal lipid component of myelin. SapA is known to bind lipids and detergents in a pH-dependent manner; this is accompanied by a striking transition from a `closed' to an `open' conformation. However, previous structures were determined at non-lysosomal pH. This work describes a 1.8 Å resolution X-ray crystal structure determined at the physiologically relevant lysosomal pH 4.8. In the absence of lipid or detergent at pH 4.8, SapA is observeed to adopt a conformation closely resembling the previously determined `closed' conformation, showing that pH alone is not sufficient for the transition to the `open' conformation. Structural alignments reveal small conformational changes, highlighting regions of flexibility.
- Department of Haematology, Cambridge Institute for Medical Research, University of Cambridge, Wellcome Trust/MRC Building, Cambridge Biomedical Campus, Hills Road, Cambridge CB2 0XY, England.
Organizational Affiliation: