Insights into the potential function and membrane organization of the TP0435 (Tp17) lipoprotein from Treponema pallidum derived from structural and biophysical analyses.
Brautigam, C.A., Deka, R.K., Liu, W.Z., Norgard, M.V.(2015) Protein Sci 24: 11-19
- PubMed: 25287511
- DOI: https://doi.org/10.1002/pro.2576
- Primary Citation of Related Structures:
4U3Q - PubMed Abstract:
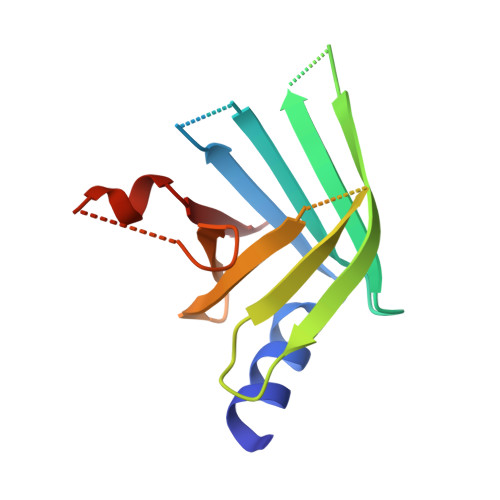
The sexually transmitted disease syphilis is caused by the bacterial spirochete Treponema pallidum. This microorganism is genetically intractable, accounting for the large number of putative and undercharacterized members of the pathogen's proteome. In an effort to ascribe a function(s) to the TP0435 (Tp17) lipoprotein, we engineered a soluble variant of the protein (rTP0435) and determined its crystal structure at a resolution of 2.42 Å. The structure is characterized by an eight-stranded β-barrel protein with a shallow "basin" at one end of the barrel and an α-helix stacked on the opposite end. Furthermore, there is a disulfide-linked dimer of the protein in the asymmetric unit of the crystals. Solution hydrodynamic experiments established that purified rTP0435 is monomeric, but specifically forms the disulfide-stabilized dimer observed in the crystal structure. The data herein, when considered with previous work on TP0435, imply plausible roles for the protein in either ligand binding, treponemal membrane architecture, and/or pathogenesis.
- Department of Biophysics, The University of Texas Southwestern Medical Center, Dallas, Texas, 75390.
Organizational Affiliation: