Structure of BACE1 complex with an anti-HMC-type inhibitor
Akaji, K., Teruya, K., Akiyama, T., Sanjho, A., Yamashita, E., Nakagawa, A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Beta-secretase 1 | 388 | Homo sapiens | Mutation(s): 0 Gene Names: BACE1, BACE, KIAA1149 EC: 3.4.23.46 | 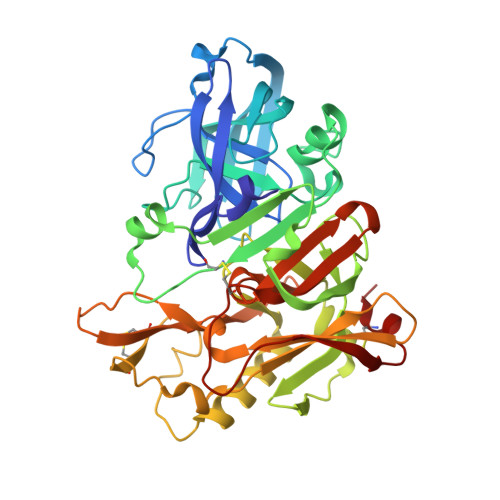 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P56817 (Homo sapiens) Explore P56817 Go to UniProtKB: P56817 | |||||
PHAROS: P56817 GTEx: ENSG00000186318 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P56817 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| GLU-ILE-TIH-THC-NVA | 4 | synthetic construct | Mutation(s): 0 | 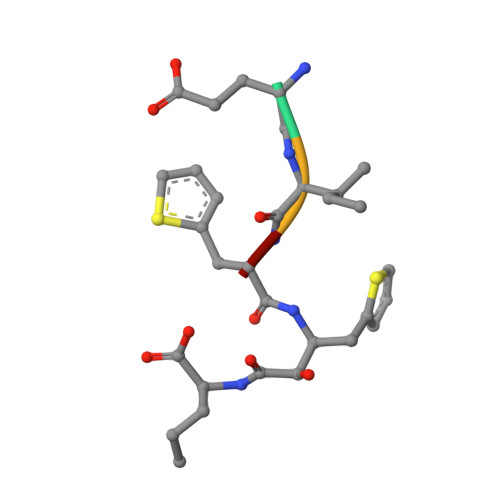 | |
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| TIH Query on TIH | D, E, F | L-PEPTIDE LINKING | C7 H9 N O2 S |  | ALA |
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_002106 Query on PRD_002106 | D, E, F | syn-HEA type inhibitor | Peptide-like / Enzyme inhibitor |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 82.326 | α = 90 |
| b = 102.618 | β = 103.53 |
| c = 101.287 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data collection |
| SCALEPACK | data reduction |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| MOLREP | model building |
| DENZO | data reduction |
| SCALEPACK | data scaling |
| MOLREP | phasing |