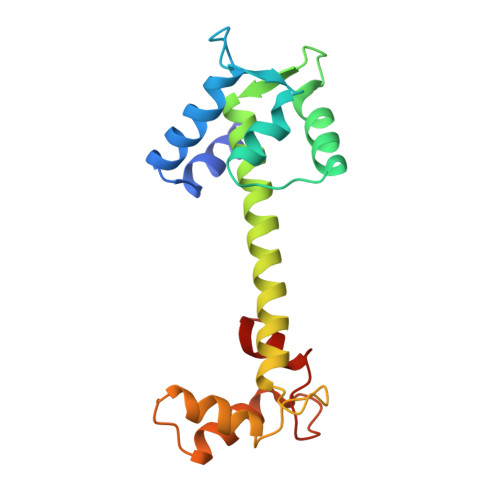
Refined structure of chicken skeletal muscle troponin C in the two-calcium state at 2-A resolution.
Satyshur, K.A., Rao, S.T., Pyzalska, D., Drendel, W., Greaser, M., Sundaralingam, M.(1988) J Biological Chem 263: 1628-1647
- PubMed: 3338985
- Primary Citation of Related Structures:
4TNC - PubMed Abstract:
The structure of troponin C has been refined at 2A resolution to an R value of 0.172 using a total of 8,100 reflections. Troponin C has an unusual dumbbell shape with only the two C-domain high affinity sites III and IV occupied with metals, while the pair of N-domain low affinity sites I and II are devoid of metals. The coordination of the Ca2+ approaches seven with the last glutamic acid residue in each site forming an asymmetric bidentate ligand. The flanking helices in the metal-bound EF hands are in similar orientation (both 113 degrees) while in the apo sites they are more obtuse (134 and 149 degrees). The EF hands of holo sites III and IV are similar while the apo sites I and II are less similar (rms for backbone atoms, 0.78 and 1.44). The half-loops of the 12-residue holo and apo sites show better agreement than the full loops themselves, suggesting a hinge motion at the midpoint of the loops. The long central helix is stabilized by electrostatic interactions and salt bridges between charged side chains spaced at 3 or 4 residues along the helix. A cluster of water molecules encircle the long helix and hydrogen bond to the backbone carbonyls. At the beginning of the B-helix, a water molecule is interposed at each of two consecutive backbone NH...OC hydrogen bonds. The terminal pair of helices A/D (apo) match with E/H (holo), and the internal pair of helices B/C (apo) match with F/G (holo). Thus, muscle contraction may be triggered by Ca2+ binding to loops I and II which results in a concerted rearrangement of residues in the loops, including the essential Gly at position 6 in each loop. This rearrangement than causes a reorientation of helices B and C along with the BC linker.
- Department of Biochemistry, College of Agriculture and Life Sciences, University of Wisconsin, Madison 53706.
Organizational Affiliation: