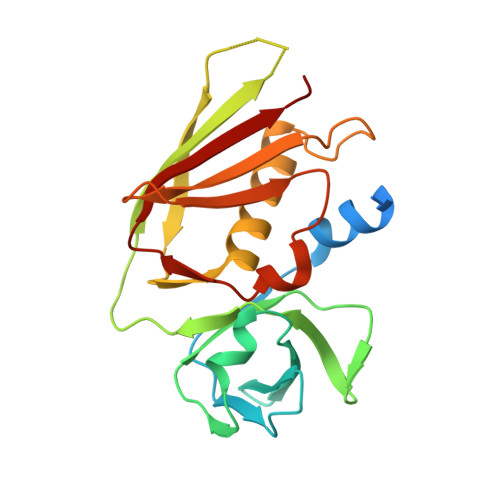
2.0 Angstrom Crystal Structure of Superantigen-like Protein from Staphylococcus aureus in Complex with 3-N-Acetylneuraminyl-N-acetyllactosamine.
Minasov, G., Nocadello, S., Shuvalova, L., Filippova, E.V., Halavaty, A., Dubrovska, I., Bagnoli, F., Falugi, F., Bottomley, M., Grandi, G., Anderson, W.F., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.