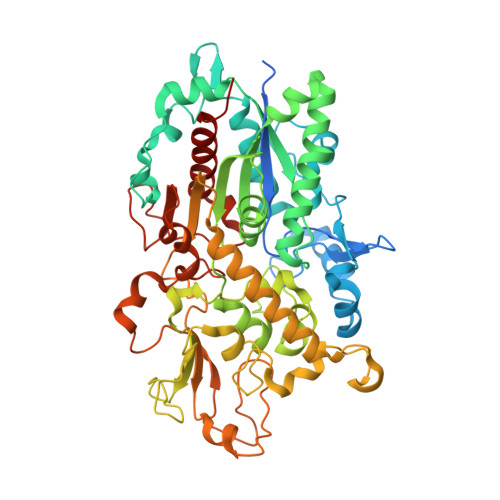
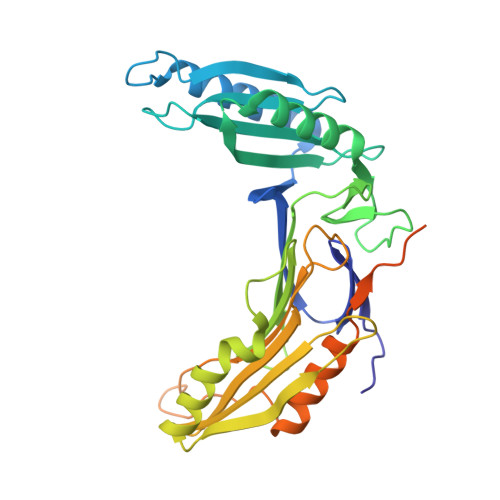
The structural basis of the Tle4-Tli4 complex reveals the self-protection mechanism of H2-T6SS in Pseudomonas aeruginosa.
Lu, D., Zheng, Y., Liao, N., Wei, L., Xu, B., Liu, X., Liu, J.(2014) Acta Crystallogr D Biol Crystallogr 70: 3233-3243
- PubMed: 25478841
- DOI: https://doi.org/10.1107/S1399004714023967
- Primary Citation of Related Structures:
4R1D - PubMed Abstract:
The type VI secretion system (T6SS) has recently been demonstrated to mediate interbacterial competition and to discriminate between self and nonself. T6SS(+) bacteria employ toxic effectors to inhibit rival cells and concurrently use effector cognate immunity proteins to protect their sibling cells. The effector and immunity pairs (E-I pairs) endow the bacteria with a great advantage in niche competition. Tle4-Tli4 (PA1510-PA1509) is a newly identified E-I pair that is controlled by H2-T6SS in Pseudomonas aeruginosa. Tle4 exhibits phospholipase activity, which destroys the cell membrane of rival cells, and the periplasm-located Tli4 in donor cells eliminates this toxic effect of Tle4. In this paper, the structure of the Tle4-Tli4 complex is reported at 1.75 Å resolution. Tle4 consists of two domains: a conserved α/β-hydrolase domain and an unusual cap domain in which two lid regions (lid1 and lid2) display a closed conformation that buries the catalytic triad in a deep funnel. Tli4 also displays a two-domain structure, in which a large lobe and a small lobe form a crab claw-like conformation. Tli4 uses this crab claw to grasp the cap domain of Tle4, especially the lid2 region, which prevents the interfacial activation of Tle4 and thus causes enzymatic dysfunction of Tle4 in sister cells.
- The United Innovation of Mengchao Hepatobiliary Technology Key Laboratory of Fujian Province, MengChao Hepatobiliary Hospital of Fujian Medical University, Fuzhou 350025, People's Republic of China.
Organizational Affiliation: