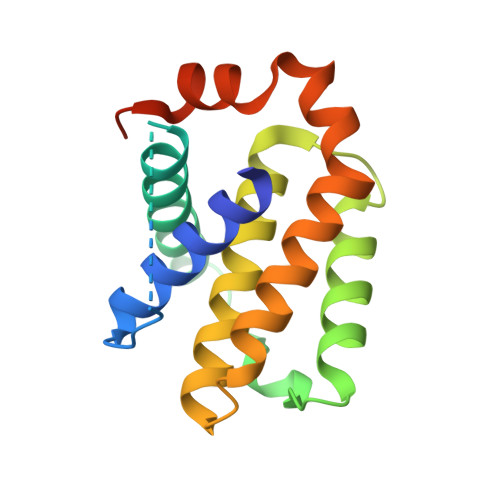
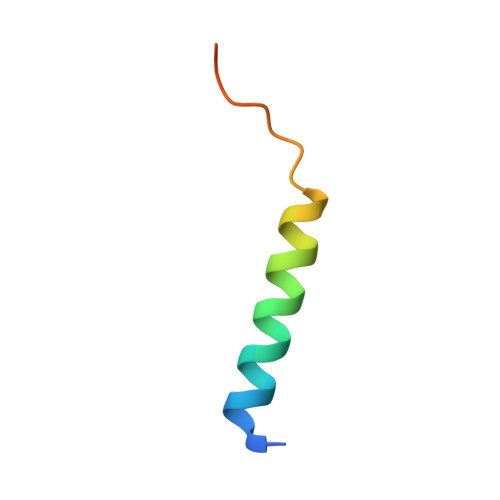
Bh3 induced conformational changes in Bcl-Xl revealed by crystal structure and comparative analysis.
Rajan, S., Choi, M., Baek, K., Yoon, H.S.(2015) Proteins 83: 1262-1272
- PubMed: 25907960
- DOI: https://doi.org/10.1002/prot.24816
- Primary Citation of Related Structures:
4QVE, 4QVF - PubMed Abstract:
Apoptosis or programmed cell death is a regulatory process in cells in response to stimuli perturbing physiological conditions. The Bcl-2 family of proteins plays an important role in regulating homeostasis during apoptosis. In the process, the molecular interactions among the three members of this family, the pro-apoptotic, anti-apoptotic and BH3-only proteins at the mitochondrial outer membrane define the fate of a cell. Here, we report the crystal structures of the human anti-apoptotic protein Bcl-XL in complex with BH3-only BID(BH3) and BIM(BH3) peptides determined at 2.0 Å and 1.5 Å resolution, respectively. The BH3 peptides bind to the canonical hydrophobic pocket in Bcl-XL and adopt an alpha helical conformation in the bound form. Despite a similar structural fold, a comparison with other BH3 complexes revealed structural differences due to their sequence variations. In the Bcl-XL-BID(BH3) complex we observed a large pocket, in comparison with other BH3 complexes, lined by residues from helices α1, α2, α3, and α5 located adjacent to the canonical hydrophobic pocket. These results suggest that there are differences in the mode of interactions by the BH3 peptides that may translate into functional differences in apoptotic regulation.
- School of Biological Sciences, Nanyang Technological University, Singapore, 637551, Singapore.
Organizational Affiliation: