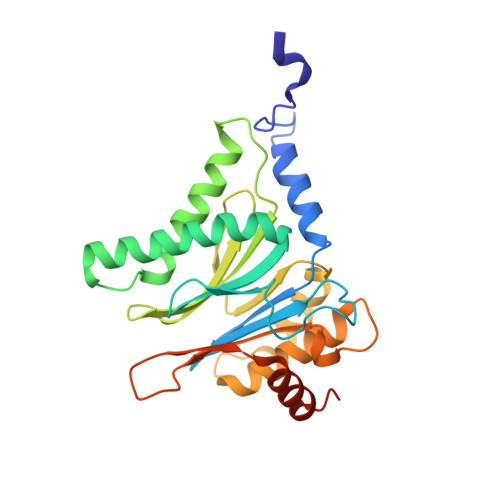
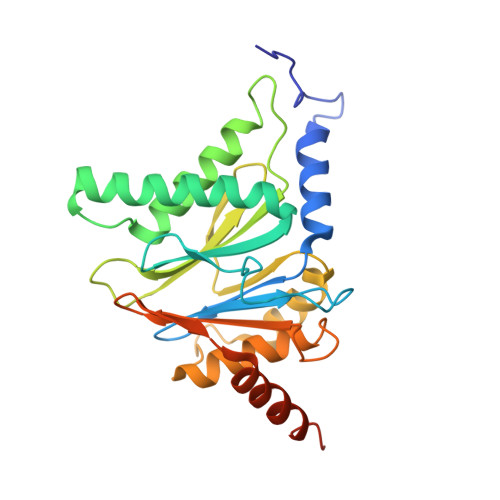
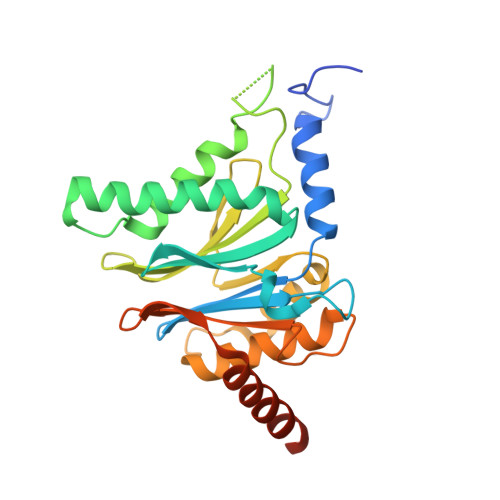
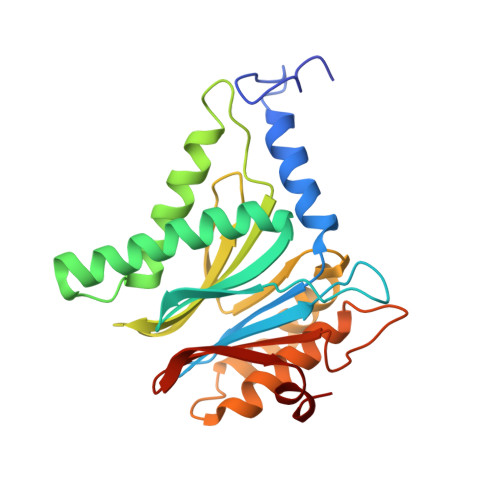
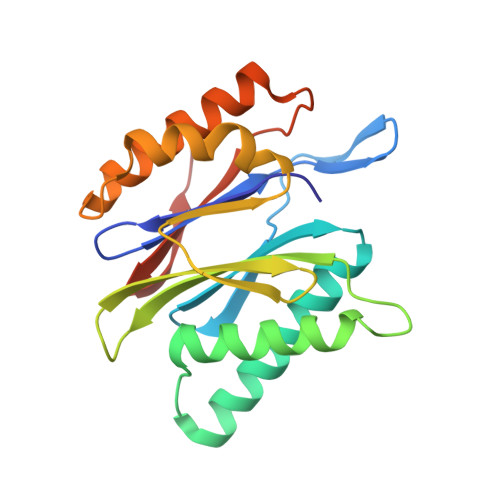
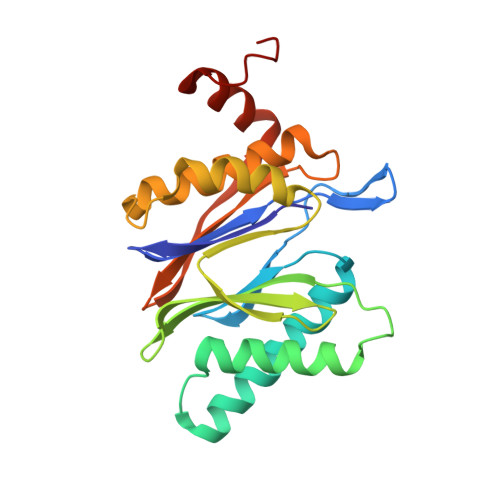
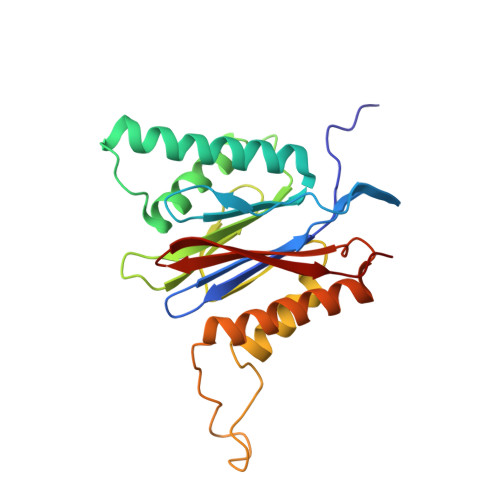
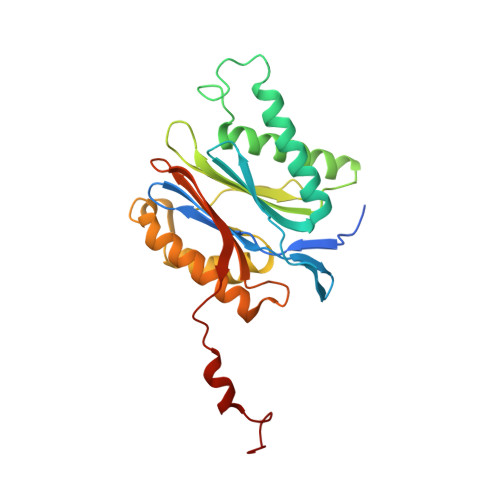
Differential global structural changes in the core particle of yeast and mouse proteasome induced by ligand binding.
Arciniega, M., Beck, P., Lange, O.F., Groll, M., Huber, R.(2014) Proc Natl Acad Sci U S A 111: 9479-9484
- PubMed: 24979800
- DOI: https://doi.org/10.1073/pnas.1408018111
- Primary Citation of Related Structures:
4QBY - PubMed Abstract:
Two clusters of configurations of the main proteolytic subunit β5 were identified by principal component analysis of crystal structures of the yeast proteasome core particle (yCP). The apo-cluster encompasses unliganded species and complexes with nonpeptidic ligands, and the pep-cluster comprises complexes with peptidic ligands. The murine constitutive CP structures conform to the yeast system, with the apo-form settled in the apo-cluster and the PR-957 (a peptidic ligand) complex in the pep-cluster. In striking contrast, the murine immune CP classifies into the pep-cluster in both the apo and the PR-957-liganded species. The two clusters differ essentially by multiple small structural changes and a domain motion enabling enclosure of the peptidic ligand and formation of specific hydrogen bonds in the pep-cluster. The immune CP species is in optimal peptide binding configuration also in its apo form. This favors productive ligand binding and may help to explain the generally increased functional activity of the immunoproteasome. Molecular dynamics simulations of the representative murine species are consistent with the experimentally observed configurations. A comparison of all 28 subunits of the unliganded species with the peptidic liganded forms demonstrates a greatly enhanced plasticity of β5 and suggests specific signaling pathways to other subunits.
- Emeritus Group Structure Research, Max Planck Institut für Biochemie, 82152 Martinsried, Germany;Center for Integrated Protein Science at the Department Chemie, Lehrstuhl für Biochemie, Technische Unversität München, 85748 Garching, Germany; castro@biochem.mpg.de huber@biochem.mpg.de.
Organizational Affiliation: