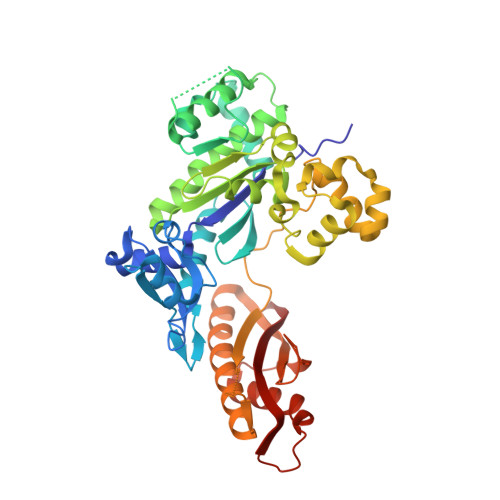
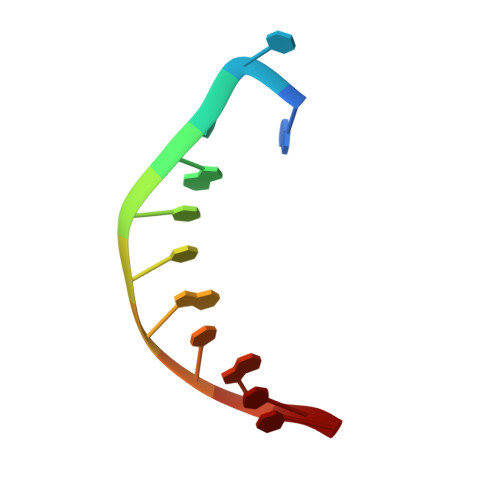
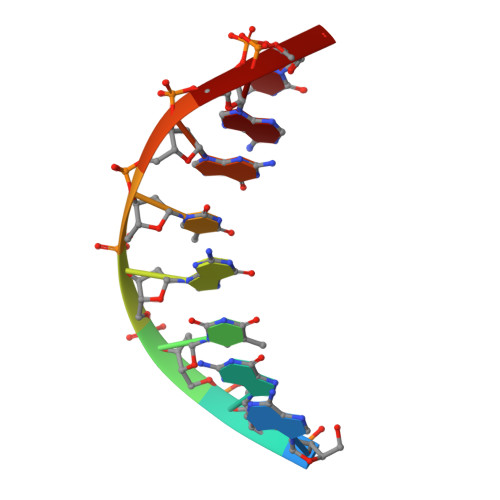
Structural and mechanistic studies of polymerase eta bypass of phenanthriplatin DNA damage.
Gregory, M.T., Park, G.Y., Johnstone, T.C., Lee, Y.S., Yang, W., Lippard, S.J.(2014) Proc Natl Acad Sci U S A 111: 9133-9138
- PubMed: 24927576
- DOI: https://doi.org/10.1073/pnas.1405739111
- Primary Citation of Related Structures:
4Q8E, 4Q8F - PubMed Abstract:
Platinum drugs are a mainstay of anticancer chemotherapy. Nevertheless, tumors often display inherent or acquired resistance to platinum-based treatments, prompting the search for new compounds that do not exhibit cross-resistance with current therapies. Phenanthriplatin, cis-diamminephenanthridinechloroplatinum(II), is a potent monofunctional platinum complex that displays a spectrum of activity distinct from those of the clinically approved platinum drugs. Inhibition of RNA polymerases by phenanthriplatin lesions has been implicated in its mechanism of action. The present study evaluates the ability of phenanthriplatin lesions to inhibit DNA replication, a function disrupted by traditional platinum drugs. Phenanthriplatin lesions effectively inhibit DNA polymerases ν, ζ, and κ and the Klenow fragment. In contrast to results obtained with DNA damaged by cisplatin, all of these polymerases were capable of inserting a base opposite a phenanthriplatin lesion, but only Pol η, an enzyme efficient in translesion synthesis, was able to fully bypass the adduct, albeit with low efficiency. X-ray structural characterization of Pol η complexed with site-specifically platinated DNA at both the insertion and +1 extension steps reveals that phenanthriplatin on DNA interacts with and inhibits Pol η in a manner distinct from that of cisplatin-DNA adducts. Unlike cisplatin and oxaliplatin, the efficacies of which are influenced by Pol η expression, phenanthriplatin is highly toxic to both Pol η+ and Pol η- cells. Given that increased expression of Pol η is a known mechanism by which cells resist cisplatin treatment, phenanthriplatin may be valuable in the treatment of cancers that are, or can easily become, resistant to cisplatin.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases andThe Johns Hopkins University-National Institutes of Health Graduate Partnership Program, National Institutes of Health, Bethesda, MD 20892; and.
Organizational Affiliation: