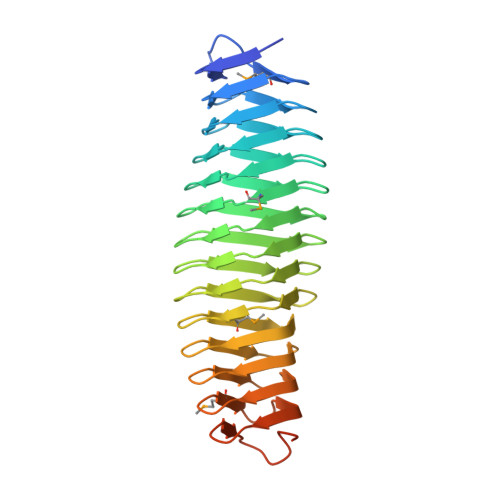
An iron-containing dodecameric heptosyltransferase family modifies bacterial autotransporters in pathogenesis
Lu, Q., Yao, Q., Xu, Y., Li, L., Li, S., Liu, Y., Gao, W., Niu, M., Sharon, M., Ben-Nissan, G., Zamyatina, A., Liu, X., Chen, S., Shao, F.(2014) Cell Host Microbe 16: 351-363
- PubMed: 25211077
- DOI: https://doi.org/10.1016/j.chom.2014.08.008
- Primary Citation of Related Structures:
4Q1Q - PubMed Abstract:
Autotransporters deliver virulence factors to the bacterial surface by translocating an effector passenger domain through a membrane-anchored barrel structure. Although passenger domains are diverse, those found in enteric bacteria autotransporters, including AIDA-I in diffusely adhering Escherichia coli (DAEC) and TibA in enterotoxigenic E. coli, are commonly glycosylated. We show that AIDA-I is heptosylated within the bacterial cytoplasm by autotransporter adhesin heptosyltransferase (AAH) and its paralogue AAH2. AIDA-I heptosylation determines DAEC adhesion to host cells. AAH/AAH2 define a bacterial autotransporter heptosyltransferase (BAHT) family that contains ferric ion and adopts a dodecamer assembly. Structural analyses of the heptosylated TibA passenger domain reveal 35 heptose conjugates forming patterned and solenoid-like arrays on the surface of a β helix. Additionally, CARC, the AIDA-like autotransporter from Citrobacter rodentium, is essential for colonization in mice and requires heptosylation by its cognate BAHT. Our study establishes a bacterial glycosylation system that regulates virulence and is essential for pathogenesis.
- National Institute of Biological Sciences, Beijing 102206, China.
Organizational Affiliation: