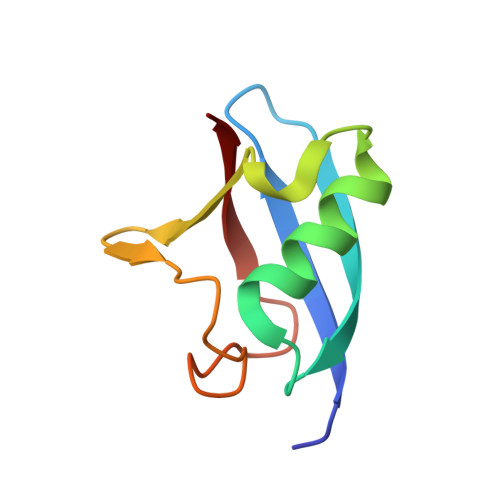
The conserved ubiquitin-like protein Hub1 plays a critical role in splicing in human cells.
Ammon, T., Mishra, S.K., Kowalska, K., Popowicz, G.M., Holak, T.A., Jentsch, S.(2014) J Mol Cell Biol 6: 312-323
- PubMed: 24872507
- DOI: https://doi.org/10.1093/jmcb/mju026
- Primary Citation of Related Structures:
4PYU - PubMed Abstract:
Different from canonical ubiquitin-like proteins, Hub1 does not form covalent conjugates with substrates but binds proteins non-covalently. In Saccharomyces cerevisiae, Hub1 associates with spliceosomes and mediates alternative splicing of SRC1, without affecting pre-mRNA splicing generally. Human Hub1 is highly similar to its yeast homolog, but its cellular function remains largely unexplored. Here, we show that human Hub1 binds to the spliceosomal protein Snu66 as in yeast; however, unlike its S. cerevisiae homolog, human Hub1 is essential for viability. Prolonged in vivo depletion of human Hub1 leads to various cellular defects, including splicing speckle abnormalities, partial nuclear retention of mRNAs, mitotic catastrophe, and consequently cell death by apoptosis. Early consequences of Hub1 depletion are severe splicing defects, however, only for specific splice sites leading to exon skipping and intron retention. Thus, the ubiquitin-like protein Hub1 is not a canonical spliceosomal factor needed generally for splicing, but rather a modulator of spliceosome performance and facilitator of alternative splicing.
- Department of Molecular Cell Biology, Max Planck Institute of Biochemistry, Am Klopferspitz 18, 82152 Martinsried, Germany.
Organizational Affiliation: