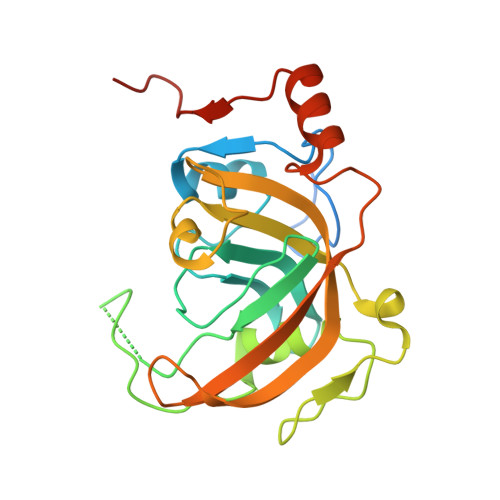
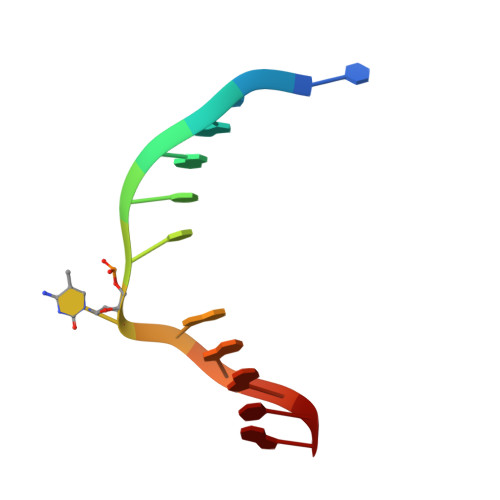
Structural Basis for Hydroxymethylcytosine Recognition by the SRA Domain of UHRF2.
Zhou, T., Xiong, J., Wang, M., Yang, N., Wong, J., Zhu, B., Xu, R.M.(2014) Mol Cell 54: 879-886
- PubMed: 24813944
- DOI: https://doi.org/10.1016/j.molcel.2014.04.003
- Primary Citation of Related Structures:
4PW5, 4PW6, 4PW7 - PubMed Abstract:
Methylated cytosine of CpG dinucleotides in vertebrates may be oxidized by Tet proteins, a process that can lead to DNA demethylation. The predominant oxidation product, 5-hydroxymethylcytosine (5hmC), has been implicated in embryogenesis, cell differentiation, and human diseases. Recently, the SRA domain of UHRF2 (UHRF2-SRA) has been reported to specifically recognize 5hmC, but how UHRF2 recognizes this modification is unclear. Here we report the structure of UHRF2-SRA in complex with a 5hmC-containing DNA. The structure reveals that the conformation of a phenylalanine allows the formation of an optimal 5hmC binding pocket, and a hydrogen bond between the hydroxyl group of 5hmC and UHRF2-SRA is critical for their preferential binding. Further structural and biochemical analyses unveiled the role of SRA domains as a versatile reader of modified DNA, and the knowledge should facilitate further understanding of the biological function of UHRF2 and the comprehension of DNA hydroxymethylation in general.
- National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Organizational Affiliation: