Structural basis of IFN receptor recognition by TYK2
Wallweber, H.J.A., Tam, C., Franke, Y., Starovasnik, M.A., Lupardus, P.J.(2014) Nat Struct Mol Biol
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2014) Nat Struct Mol Biol
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Non-receptor tyrosine-protein kinase TYK2 | 567 | Homo sapiens | Mutation(s): 0 Gene Names: TYK2 EC: 2.7.10.2 | 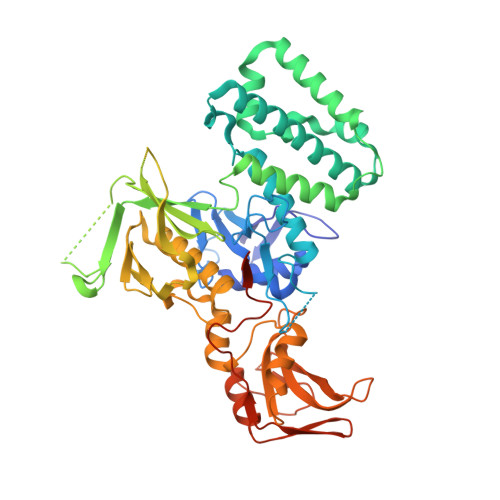 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P29597 (Homo sapiens) Explore P29597 Go to UniProtKB: P29597 | |||||
PHAROS: P29597 GTEx: ENSG00000105397 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P29597 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Interferon alpha/beta receptor 1 | 34 | Homo sapiens | Mutation(s): 0 Gene Names: IFNAR, IFNAR1 | 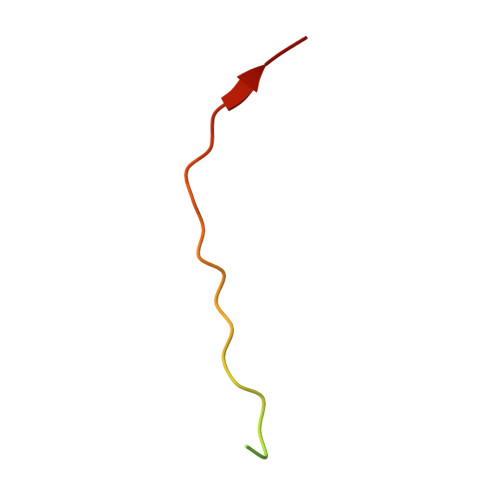 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P17181 (Homo sapiens) Explore P17181 Go to UniProtKB: P17181 | |||||
PHAROS: P17181 GTEx: ENSG00000142166 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P17181 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GOL Query on GOL | C [auth A], D [auth A], E [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 186.768 | α = 90 |
| b = 52.422 | β = 108.09 |
| c = 70.163 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SSRL | data collection |
| SOLVE | phasing |
| BUSTER | refinement |
| HKL-2000 | data reduction |
| SCALA | data scaling |