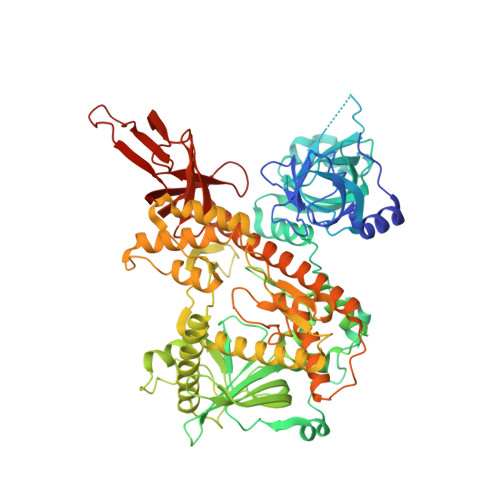
Mechanism of Dis3l2 substrate recognition in the Lin28-let-7 pathway.
Faehnle, C.R., Walleshauser, J., Joshua-Tor, L.(2014) Nature 514: 252-256
- PubMed: 25119025
- DOI: https://doi.org/10.1038/nature13553
- Primary Citation of Related Structures:
4PMW - PubMed Abstract:
The pluripotency factor Lin28 inhibits the biogenesis of the let-7 family of mammalian microRNAs. Lin28 is highly expressed in embryonic stem cells and has a fundamental role in regulation of development, glucose metabolism and tissue regeneration. Overexpression of Lin28 is correlated with the onset of numerous cancers, whereas let-7, a tumour suppressor, silences several human oncogenes. Lin28 binds to precursor let-7 (pre-let-7) hairpins, triggering the 3' oligo-uridylation activity of TUT4 and TUT7 (refs 10-12). The oligoU tail added to pre-let-7 serves as a decay signal, as it is rapidly degraded by Dis3l2 (refs 13, 14), a homologue of the catalytic subunit of the RNA exosome. The molecular basis of Lin28-mediated recruitment of TUT4 and TUT7 to pre-let-7 and its subsequent degradation by Dis3l2 is largely unknown. To examine the mechanism of Dis3l2 substrate recognition we determined the structure of mouse Dis3l2 in complex with an oligoU RNA to mimic the uridylated tail of pre-let-7. Three RNA-binding domains form an open funnel on one face of the catalytic domain that allows RNA to navigate a path to the active site different from that of its exosome counterpart. The resulting path reveals an extensive network of uracil-specific interactions spanning the first 12 nucleotides of an oligoU-tailed RNA. We identify three U-specificity zones that explain how Dis3l2 recognizes, binds and processes uridylated pre-let-7 in the final step of the Lin28-let-7 pathway.
- W. M. Keck Structural Biology Laboratory 1 Bungtown Road, Cold Spring Harbor, NY 11724, USA.
Organizational Affiliation: