Oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad spectrum antibacterial agents.
Singh, S.B., Kaelin, D.E., Wu, J., Miesel, L., Tan, C.M., Meinke, P.T., Olsen, D., Lagrutta, A., Bradley, P., Lu, J., Patel, S., Rickert, K.W., Smith, R.F., Soisson, S., Wei, C., Fukuda, H., Kishii, R., Takei, M., Fukuda, Y.(2014) ACS Med Chem Lett 5: 609-614
- PubMed: 24900889
- DOI: https://doi.org/10.1021/ml500069w
- Primary Citation of Related Structures:
4PLB - PubMed Abstract:
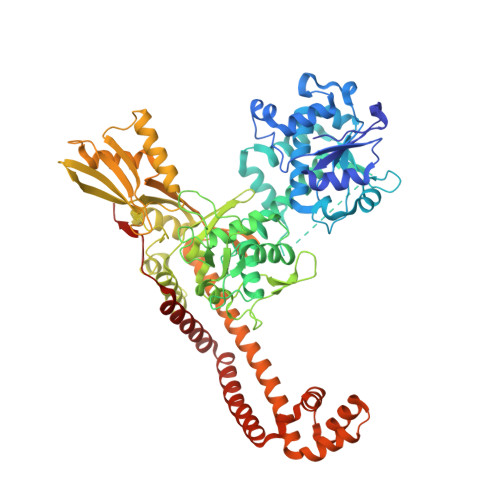
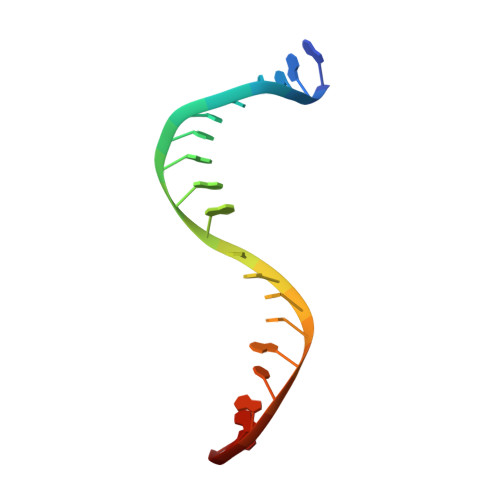
Bacterial resistance is eroding the clinical utility of existing antibiotics necessitating the discovery of new agents. Bacterial type II topoisomerase is a clinically validated, highly effective, and proven drug target. This target is amenable to inhibition by diverse classes of inhibitors with alternative and distinct binding sites to quinolone antibiotics, thus enabling the development of agents that lack cross-resistance to quinolones. Described here are novel bacterial topoisomerase inhibitors (NBTIs), which are a new class of gyrase and topo IV inhibitors and consist of three distinct structural moieties. The substitution of the linker moiety led to discovery of potent broad-spectrum NBTIs with reduced off-target activity (hERG IC50 > 18 μM) and improved physical properties. AM8191 is bactericidal and selectively inhibits DNA synthesis and Staphylococcus aureus gyrase (IC50 = 1.02 μM) and topo IV (IC50 = 10.4 μM). AM8191 showed parenteral and oral efficacy (ED50) at less than 2.5 mg/kg doses in a S. aureus murine infection model. A cocrystal structure of AM8191 bound to S. aureus DNA-gyrase showed binding interactions similar to that reported for GSK299423, displaying a key contact of Asp83 with the basic amine at position-7 of the linker.
- Merck Research Laboratories , Kenilworth, New Jersey 07033, United States.
Organizational Affiliation: