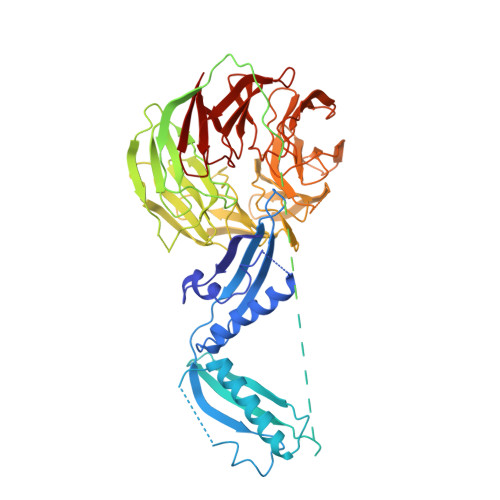
Crystal Structure of BamB Bound to a Periplasmic Domain Fragment of BamA, the Central Component of the beta-Barrel Assembly Machine.
Jansen, K.B., Baker, S.L., Sousa, M.C.(2015) J Biological Chem 290: 2126-2136
- PubMed: 25468906
- DOI: https://doi.org/10.1074/jbc.M114.584524
- Primary Citation of Related Structures:
4PK1 - PubMed Abstract:
The β-barrel assembly machinery (BAM) mediates folding and insertion of β-barrel outer membrane proteins (OMPs) into the outer membrane of Gram-negative bacteria. BAM is a five-protein complex consisting of the β-barrel OMP BamA and lipoproteins BamB, -C, -D, and -E. High resolution structures of all the individual BAM subunits and a BamD-BamC complex have been determined. However, the overall complex architecture remains elusive. BamA is the central component of BAM and consists of a membrane-embedded β-barrel and a periplasmic domain with five polypeptide translocation-associated (POTRA) motifs thought to interact with the accessory lipoproteins. Here we report the crystal structure of a fusion between BamB and a POTRA3-5 fragment of BamA. Extended loops 13 and 17 protruding from one end of the BamB β-propeller contact the face of the POTRA3 β-sheet in BamA. The interface is stabilized by several hydrophobic contacts, a network of hydrogen bonds, and a cation-π interaction between BamA Tyr-255 and BamB Arg-195. Disruption of BamA-BamB binding by BamA Y255A and probing of the interface by disulfide bond cross-linking validate the physiological relevance of the observed interface. Furthermore, the structure is consistent with previously published mutagenesis studies. The periplasmic five-POTRA domain of BamA is flexible in solution due to hinge motions in the POTRA2-3 linker. Modeling BamB in complex with full-length BamA shows BamB binding at the POTRA2-3 hinge, suggesting a role in modulation of BamA flexibility and the conformational changes associated with OMP folding and insertion.
- From the Department of Chemistry and Biochemistry, University of Colorado, Boulder, Colorado 80309.
Organizational Affiliation: