Chemical constraints on the radical cation center of cytochrome c peroxidase for long-range electron transfer
Crane, B.R., Yee, E.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cytochrome c peroxidase, mitochondrial | 294 | Saccharomyces cerevisiae S288C | Mutation(s): 1 Gene Names: CCP1, CCP, CPO, YKR066C EC: 1.11.1.5 | 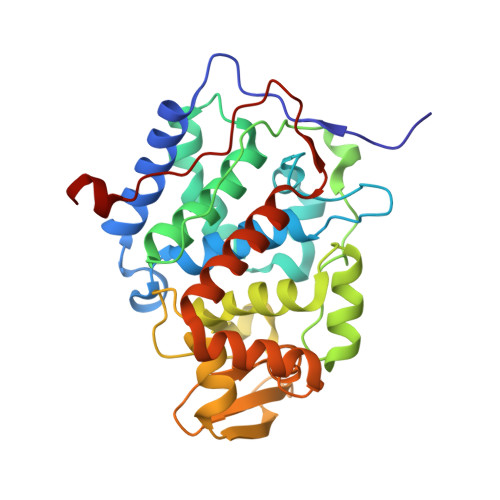 | |
UniProt | |||||
Find proteins for P00431 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P00431 Go to UniProtKB: P00431 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00431 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cytochrome c iso-1 | 103 | Saccharomyces cerevisiae S288C | Mutation(s): 0 Gene Names: CYC1, YJR048W, J1653 | 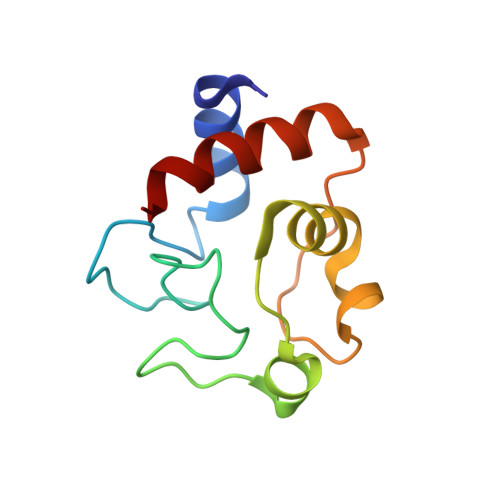 | |
UniProt | |||||
Find proteins for P00044 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P00044 Go to UniProtKB: P00044 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00044 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HEM Query on HEM | E [auth A], F [auth B], G [auth C], H [auth D] | PROTOPORPHYRIN IX CONTAINING FE C34 H32 Fe N4 O4 KABFMIBPWCXCRK-RGGAHWMASA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 44.797 | α = 90 |
| b = 113.842 | β = 105.31 |
| c = 88.239 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHASER | phasing |
| PDB_EXTRACT | data extraction |
| PHENIX | refinement |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Science Foundation (NSF, United States) | United States | CHE-0749997 |