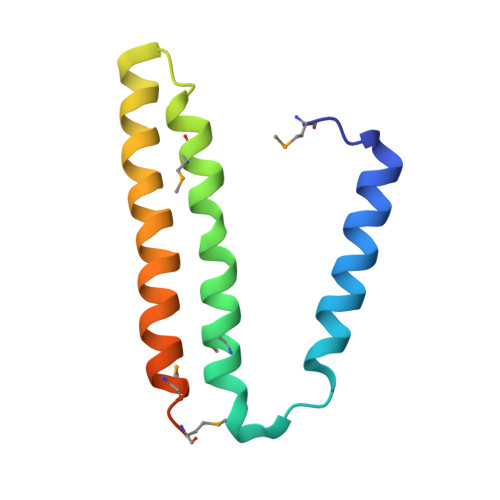
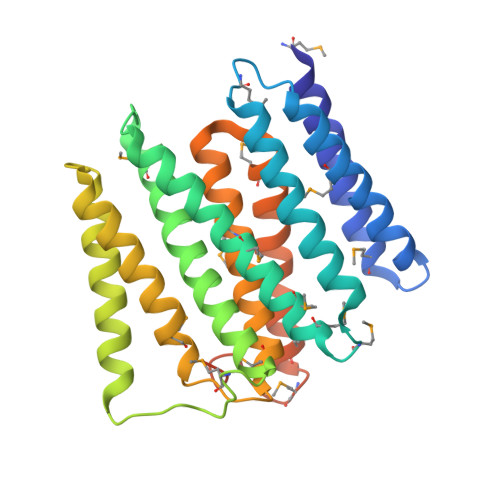
Structural biology. Division of labor in transhydrogenase by alternating proton translocation and hydride transfer.
Leung, J.H., Schurig-Briccio, L.A., Yamaguchi, M., Moeller, A., Speir, J.A., Gennis, R.B., Stout, C.D.(2015) Science 347: 178-181
- PubMed: 25574024
- DOI: https://doi.org/10.1126/science.1260451
- Primary Citation of Related Structures:
4O93, 4O9P, 4O9T, 4O9U - PubMed Abstract:
NADPH/NADP(+) (the reduced form of NADP(+)/nicotinamide adenine dinucleotide phosphate) homeostasis is critical for countering oxidative stress in cells. Nicotinamide nucleotide transhydrogenase (TH), a membrane enzyme present in both bacteria and mitochondria, couples the proton motive force to the generation of NADPH. We present the 2.8 Å crystal structure of the transmembrane proton channel domain of TH from Thermus thermophilus and the 6.9 Å crystal structure of the entire enzyme (holo-TH). The membrane domain crystallized as a symmetric dimer, with each protomer containing a putative proton channel. The holo-TH is a highly asymmetric dimer with the NADP(H)-binding domain (dIII) in two different orientations. This unusual arrangement suggests a catalytic mechanism in which the two copies of dIII alternatively function in proton translocation and hydride transfer.
- Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.
Organizational Affiliation: