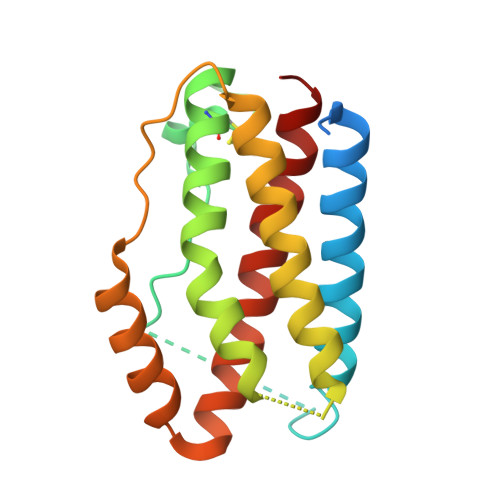
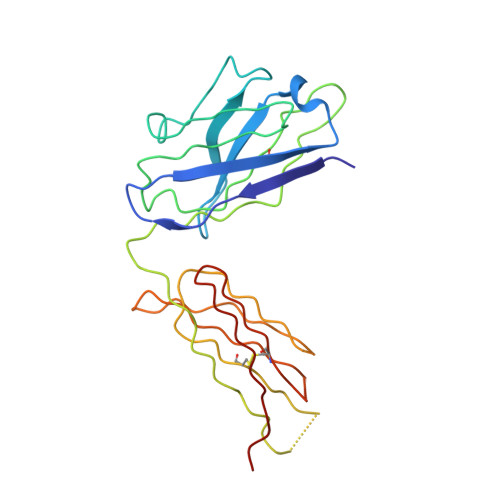
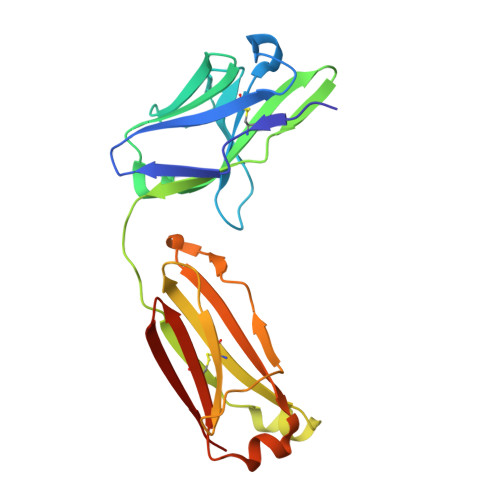
Combining residues of naturally-occurring Camelid somatic affinity variants yields ultra-potent human therapeutic IL-6 antibodies
Klarenbeek, A., Blanchetot, C., Schragel, G., Sadi, A.S., Ongenae, N., Hemrika, W., Wijdenes, J., Spinelli, S., Desmyter, A., Cambillau, C., Hultberg, A., Kretz-Rommel, A., Dreier, T., De haard, H.J.W., Roovers, R.C.To be published.