Structural and Functional Analysis of the GerD Spore Germination Protein of Bacillus Species.
Li, Y., Jin, K., Ghosh, S., Devarakonda, P., Carlson, K., Davis, A., Stewart, K.A., Cammett, E., Rossi, P.P., Setlow, B., Lu, M., Setlow, P., Hao, B.(2014) J Mol Biology 426: 1995-2008
- PubMed: 24530795
- DOI: https://doi.org/10.1016/j.jmb.2014.02.004
- Primary Citation of Related Structures:
4O8W - PubMed Abstract:
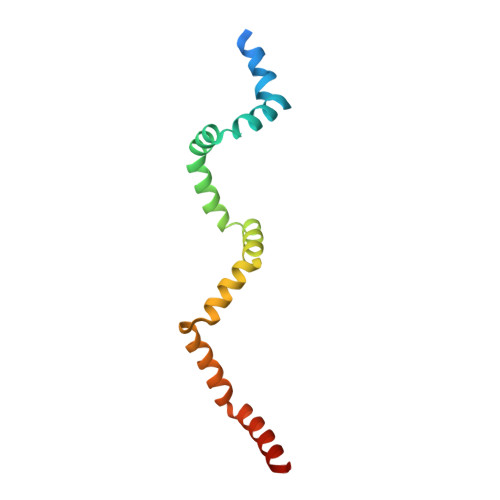
Spore germination in Bacillus species represents an excellent model system with which to study the molecular mechanisms underlying the nutritional control of growth and development. Binding of specific chemical nutrients to their cognate receptors located in the spore inner membrane triggers the germination process that leads to a resumption of metabolism in spore outgrowth. Recent studies suggest that the inner membrane GerD lipoprotein plays a critical role in the receptor-mediated activation of downstream germination events. The 121-residue core polypeptide of GerD (GerD⁶⁰⁻¹⁸⁰) from Geobacillus stearothermophilus forms a stable α-helical trimer in aqueous solution. The 2.3-Å-resolution crystal structure of the trimer reveals a neatly twisted superhelical rope, with unusual supercoiling induced by parallel triple-helix interactions. The overall geometry comprises three interleaved hydrophobic screws of interacting helices linked by short turns that have not been seen before. Using complementation analysis in a series of Bacillus subtilis gerD mutants, we demonstrated that alterations in the GerD trimer structure have profound effects on nutrient germination. This important structure-function relationship of trimeric GerD is supported by our identification of a dominant negative gerD mutation in B. subtilis. These results and those of others lead us to propose that GerD mediates clustering of germination proteins in the inner membrane of dormant spores and thus promotes the rapid and cooperative germination response to nutrients.
- Department of Molecular Biology and Biophysics, University of Connecticut Health Center, Farmington, CT 06030-3305, USA.
Organizational Affiliation: