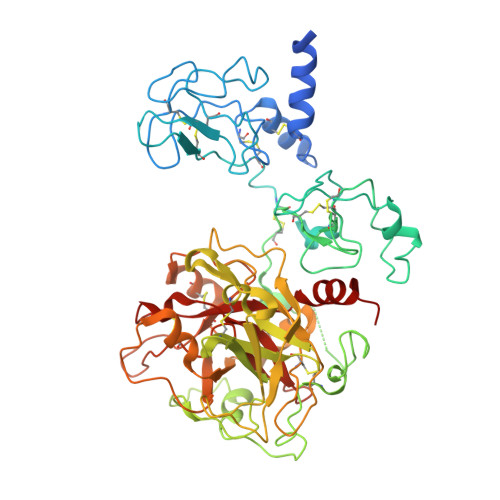
The linker connecting the two kringles plays a key role in prothrombin activation.
Pozzi, N., Chen, Z., Pelc, L.A., Shropshire, D.B., Di Cera, E.(2014) Proc Natl Acad Sci U S A 111: 7630-7635
- PubMed: 24821807
- DOI: https://doi.org/10.1073/pnas.1403779111
- Primary Citation of Related Structures:
4NZQ, 4O03 - PubMed Abstract:
The zymogen prothrombin is proteolytically converted by factor Xa to the active protease thrombin in a reaction that is accelerated >3,000-fold by cofactor Va. This physiologically important effect is paradigmatic of analogous cofactor-dependent reactions in the coagulation and complement cascades, but its structural determinants remain poorly understood. Prothrombin has three linkers connecting the N-terminal Gla domain to kringle-1 (Lnk1), the two kringles (Lnk2), and kringle-2 to the C-terminal protease domain (Lnk3). Recent developments indicate that the linkers, and particularly Lnk2, confer on the zymogen significant flexibility in solution and enable prothrombin to sample alternative conformations. The role of this flexibility in the context of prothrombin activation was tested with several deletions. Removal of Lnk2 in almost its entirety (ProTΔ146-167) drastically reduces the enhancement of thrombin generation by cofactor Va from >3,000-fold to 60-fold because of a significant increase in the rate of activation in the absence of cofactor. Deletion of Lnk2 mimics the action of cofactor Va and offers insights into how prothrombin is activated at the molecular level. The crystal structure of ProTΔ146-167 reveals a contorted architecture where the domains are not vertically stacked, kringle-1 comes within 9 Å of the protease domain, and the Gla-domain primed for membrane binding comes in contact with kringle-2. These findings broaden our molecular understanding of a key reaction of the blood coagulation cascade where cofactor Va enhances activation of prothrombin by factor Xa by compressing Lnk2 and morphing prothrombin into a conformation similar to the structure of ProTΔ146-167.
- Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, St. Louis, MO 63104.
Organizational Affiliation: