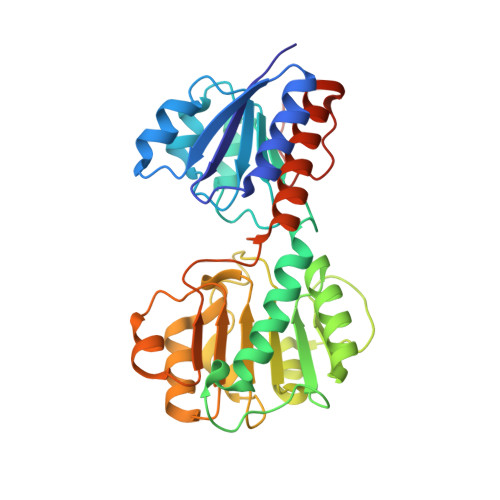
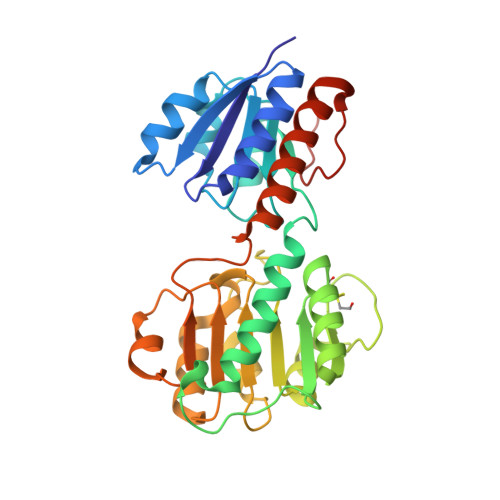
Crystal structures and kinetics of Type III 3-phosphoglycerate dehydrogenase reveal catalysis by lysine.
Singh, R.K., Raj, I., Pujari, R., Gourinath, S.(2014) FEBS J 281: 5498-5512
- PubMed: 25294608
- DOI: https://doi.org/10.1111/febs.13091
- Primary Citation of Related Structures:
4NFY, 4NJM, 4NJO - PubMed Abstract:
D-Phosphoglycerate dehydrogenase (PGDH) catalyzes the first committed step of the phosphorylated serine biosynthesis pathway. Here, we report for the first time, the crystal structures of Type IIIK PGDH from Entamoeba histolytica in the apo form, as well as in complexes with substrate (3-phosphoglyceric acid) and cofactor (NAD(+) ) to 2.45, 1.8 and 2.2 Å resolution, respectively. Comparison of the apo structure with the substrate-bound structure shows that the substrate-binding domain is rotated by ~ 20° to close the active-site cleft. The cofactor-bound structure also shows a closed-cleft conformation, in which NAD(+) is bound to the nucleotide-binding domain and a formate ion occupies the substrate-binding site. Superposition of the substrate- and cofactor-bound structures represents a snapshot of the enzyme in the active form, where C2 of the substrate and C4N of the cofactor are 2.2 Å apart, and the amino group of Lys263 is close enough to the substrate to remove the proton from the hydroxyl group of PGA, indicating the role of Lys in the catalysis. Mutation of Lys263 to Ala yields just 0.8% of the specific activity of the wild-type enzyme, revealing that Lys263 indeed plays an integral role in the catalytic activity. The detectable activity of the mutant, however, indicates that after 20° rotation of the substrate-binding domain, the resulting positions of the substrate and cofactor are sufficiently close to make a productive reaction.
- School of Life Sciences, Jawaharlal Nehru University, New Delhi, India.
Organizational Affiliation: