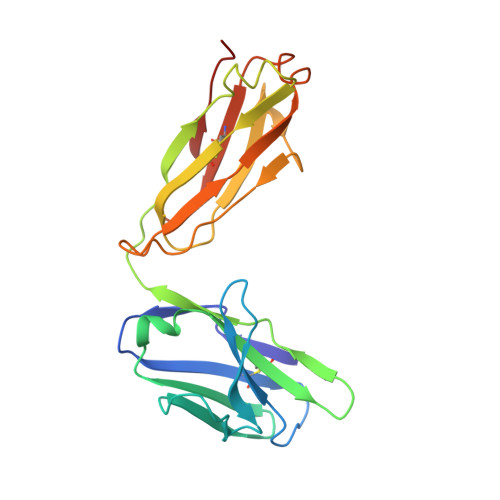
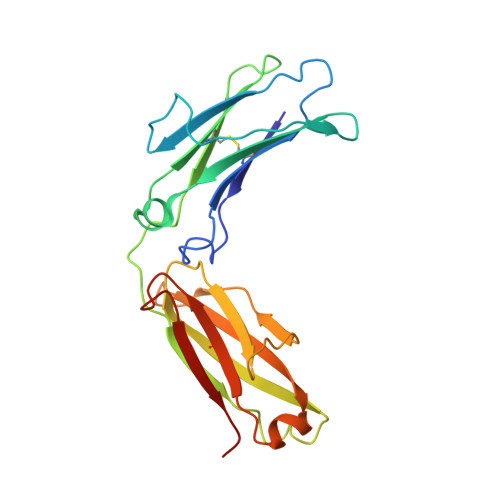
Structural basis for enhanced HIV-1 neutralization by a dimeric immunoglobulin G form of the glycan-recognizing antibody 2G12.
Wu, Y., West, A.P., Kim, H.J., Thornton, M.E., Ward, A.B., Bjorkman, P.J.(2013) Cell Rep 5: 1443-1455
- PubMed: 24316082
- DOI: https://doi.org/10.1016/j.celrep.2013.11.015
- Primary Citation of Related Structures:
4NHG, 4NHH - PubMed Abstract:
The human immunoglobulin G (IgG) 2G12 recognizes high-mannose carbohydrates on the HIV type 1 (HIV-1) envelope glycoprotein gp120. Its two antigen-binding fragments (Fabs) are intramolecularly domain exchanged, resulting in a rigid (Fab)2 unit including a third antigen-binding interface not found in antibodies with flexible Fab arms. We determined crystal structures of dimeric 2G12 IgG created by intermolecular domain exchange, which exhibits increased breadth and >50-fold increased neutralization potency compared with monomeric 2G12. The four Fab and two fragment crystalline (Fc) regions of dimeric 2G12 were localized at low resolution in two independent structures, revealing IgG dimers with two (Fab)2 arms analogous to the Fabs of conventional monomeric IgGs. Structures revealed three conformationally distinct dimers, demonstrating flexibility of the (Fab)2-Fc connections that was confirmed by electron microscopy, small-angle X-ray scattering, and binding studies. We conclude that intermolecular domain exchange, flexibility, and bivalent binding to allow avidity effects are responsible for the increased potency and breadth of dimeric 2G12.
- Division of Biology and Biological Engineering 114-96, California Institute of Technology, 1200 East California Boulevard, Pasadena, CA 91125, USA.
Organizational Affiliation: