Deciphering the role of 2-O-sulfotransferase in regulating heparan sulfate biosynthesis
Liu, C., Sheng, J., Krahn, J.M., Perera, L., Xu, Y., Hsieh, P., Liu, J., Pedersen, L.C.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Maltose-binding periplasmic protein, Heparan sulfate 2-O-sulfotransferase 1 fusion | 658 | Escherichia coli UMEA 3304-1, Gallus gallus This entity is chimeric | Mutation(s): 4 Gene Names: G962_03763, HS2ST1, HS2ST EC: 2.8.2 | 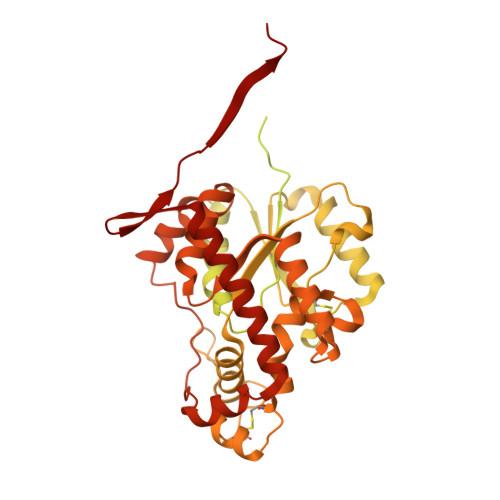 | |
UniProt | |||||
Find proteins for P0AEX9 (Escherichia coli (strain K12)) Explore P0AEX9 Go to UniProtKB: P0AEX9 | |||||
Find proteins for Q76KB1 (Gallus gallus) Explore Q76KB1 Go to UniProtKB: Q76KB1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | P0AEX9Q76KB1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | 2D Diagram | Glycosylation | 3D Interactions |
| beta-D-glucopyranuronic acid-(1-4)-2-acetamido-2-deoxy-alpha-D-glucopyranose-(1-4)-beta-D-glucopyranuronic acid-(1-4)-2-deoxy-2-(sulfoamino)-alpha-D-glucopyranose-(1-4)-alpha-L-idopyranuronic acid-(1-4)-2-deoxy-2-(sulfoamino)-alpha-D-glucopyranose-(1-4)-beta-D-glucopyranuronic acid | G, I, L, N, P | 7 |  | N/A | |
Glycosylation Resources | |||||
GlyTouCan: G54413LX GlyCosmos: G54413LX | |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| A3P Query on A3P | Q [auth A] S [auth B] U [auth C] V [auth D] X [auth E] | ADENOSINE-3'-5'-DIPHOSPHATE C10 H15 N5 O10 P2 WHTCPDAXWFLDIH-KQYNXXCUSA-N |  | ||
| NPO Query on NPO | R [auth A], T [auth B], W [auth D], Y [auth E] | P-NITROPHENOL C6 H5 N O3 BTJIUGUIPKRLHP-UHFFFAOYSA-N |  | ||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| ID | Chains | Name | Type/Class | 2D Diagram | 3D Interactions |
| PRD_900001 Query on PRD_900001 | H, J, K, M, O | alpha-maltose | Oligosaccharide / Nutrient |  | |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 155.72 | α = 90 |
| b = 170.69 | β = 90 |
| c = 183.96 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SERGUI | data collection |
| PHASER | phasing |
| PHENIX | model building |
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |