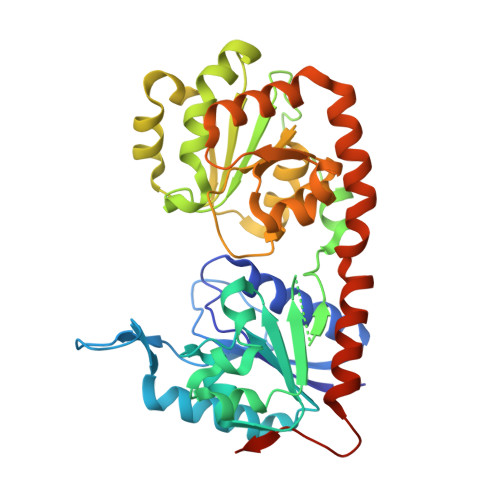
Secondary structure reshuffling modulates glycosyltransferase function at the membrane.
Giganti, D., Albesa-Jove, D., Urresti, S., Rodrigo-Unzueta, A., Martinez, M.A., Comino, N., Barilone, N., Bellinzoni, M., Chenal, A., Guerin, M.E., Alzari, P.M.(2015) Nat Chem Biol 11: 16-18
- PubMed: 25402770
- DOI: https://doi.org/10.1038/nchembio.1694
- Primary Citation of Related Structures:
4N9W, 4NC9 - PubMed Abstract:
Secondary structure refolding is a key event in biology as it modulates the conformation of many proteins in the cell, generating functional or aberrant states. The crystal structures of mannosyltransferase PimA reveal an exceptional flexibility of the protein along the catalytic cycle, including β-strand-to-α-helix and α-helix-to-β-strand transitions. These structural changes modulate catalysis and are promoted by interactions of the protein with anionic phospholipids in the membrane.
- 1] Institut Pasteur, Unité de Microbiologie Structurale, CNRS UMR 3528, Paris, France. [2] Unidad de Biofisica, Centro Mixto Consejo Superior de Investigaciones Cientificas-Universidad del País Vasco/Euskal Herriko Unibertsitatea (CSIC,UPV/EHU), Leioa, Bizkaia, Spain. [3] Departamento de Bioquímica, Universidad del País Vasco, Spain.
Organizational Affiliation: