Picomolar Inhibitors of HIV Reverse Transcriptase Featuring Bicyclic Replacement of a Cyanovinylphenyl Group.
Lee, W.G., Gallardo-Macias, R., Frey, K.M., Spasov, K.A., Bollini, M., Anderson, K.S., Jorgensen, W.L.(2013) J Am Chem Soc 135: 16705-16713
- PubMed: 24151856
- DOI: https://doi.org/10.1021/ja408917n
- Primary Citation of Related Structures:
4MFB - PubMed Abstract:
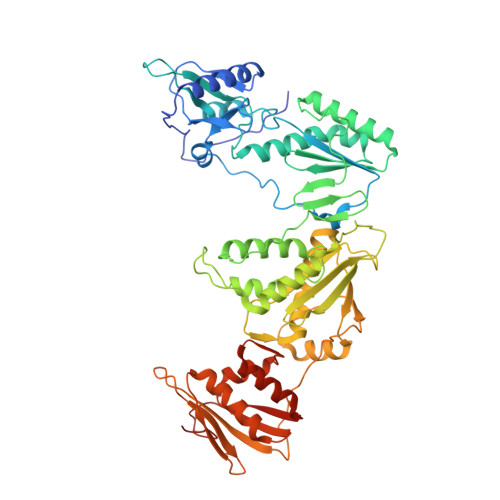
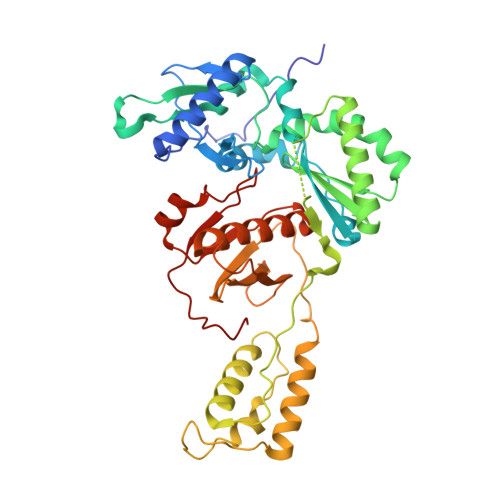
Members of the catechol diether class are highly potent non-nucleoside inhibitors of HIV-1 reverse transcriptase (NNRTIs). The most active compounds yield EC50 values below 0.5 nM in assays using human T-cells infected by wild-type HIV-1. However, these compounds such as rilpivirine, the most recently FDA-approved NNRTI, bear a cyanovinylphenyl (CVP) group. This is an uncommon substructure in drugs that gives reactivity concerns. In the present work, computer simulations were used to design bicyclic replacements for the CVP group. The predicted viability of a 2-cyanoindolizinyl alternative was confirmed experimentally and provided compounds with 0.4 nM activity against the wild-type virus. The compounds also performed well with EC50 values of 10 nM against the challenging HIV-1 variant that contains the Lys103Asn/Tyr181Cys double mutation in the RT enzyme. Indolyl and benzofuranyl analogues were also investigated; the most potent compounds in these cases have EC50 values toward wild-type HIV-1 near 10 nM and high-nanomolar activities toward the double-variant. The structural expectations from the modeling were much enhanced by obtaining an X-ray crystal structure at 2.88 Å resolution for the complex of the parent 2-cyanoindolizine 10b and HIV-1 RT. The aqueous solubilities of the most potent indolizine analogues were also measured to be ~40 μg/mL, which is similar to that for the approved drug efavirenz and ~1000-fold greater than for rilpivirine.
- Department of Chemistry, Yale University , New Haven, Connecticut 06520-8107, United States.
Organizational Affiliation: