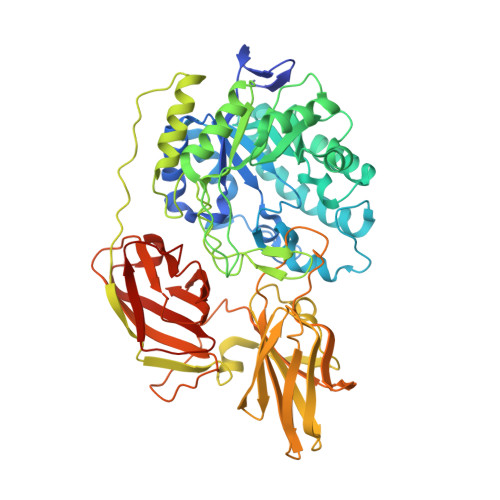
Rational design of a glycosynthase by the crystal structure of beta-galactosidase from Bacillus circulans (BgaC) and its use for the synthesis of N-acetyllactosamine type 1 glycan structures.
Henze, M., You, D.J., Kamerke, C., Hoffmann, N., Angkawidjaja, C., Ernst, S., Pietruszka, J., Kanaya, S., Elling, L.(2014) J Biotechnol 191: 78-85
- PubMed: 25034434
- DOI: https://doi.org/10.1016/j.jbiotec.2014.07.003
- Primary Citation of Related Structures:
4MAD - PubMed Abstract:
The crystal structure of β-galactosidase from Bacillus circulans (BgaC) was determined at 1.8Å resolution. The overall structure of BgaC consists of three distinct domains, which are the catalytic domain with a TIM-barrel structure and two all-β domains (ABDs). The main-chain fold and steric configurations of the acidic and aromatic residues at the active site were very similar to those of Streptococcus pneumoniae β(1,3)-galactosidase BgaC in complex with galactose. The structure of BgaC was used for the rational design of a glycosynthase. BgaC belongs to the glycoside hydrolase family 35. The essential nucleophilic amino acid residue has been identified as glutamic acid at position 233 by site-directed mutagenesis. Construction of the active site mutant BgaC-Glu233Gly gave rise to a galactosynthase transferring the sugar moiety from α-d-galactopyranosyl fluoride (αGalF) to different β-linked N-acetylglucosamine acceptor substrates in good yield (40-90%) with a remarkably stable product formation. Enzymatic syntheses with BgaC-Glu233Gly afforded the stereo- and regioselective synthesis of β1-3-linked key galactosides like galacto-N-biose or lacto-N-biose.
- Laboratory for Biomaterials, Institute of Biotechnology and Helmholtz-Institute for Biomedical Engineering, RWTH Aachen University, 52074 Aachen, Germany.
Organizational Affiliation: