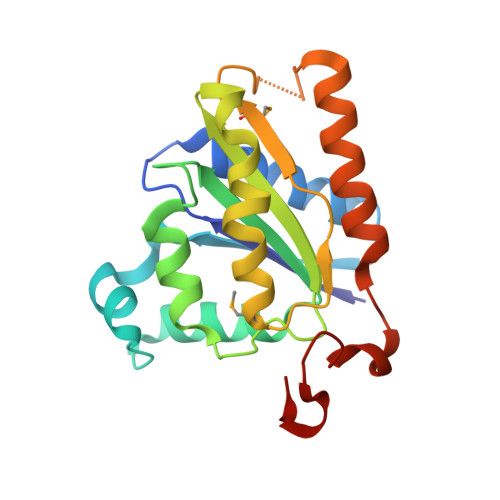
Crystal structures of NADH:FMN oxidoreductase (EmoB) at different stages of catalysis.
Nissen, M.S., Youn, B., Knowles, B.D., Ballinger, J.W., Jun, S.Y., Belchik, S.M., Xun, L., Kang, C.(2008) J Biological Chem 283: 28710-28720
- PubMed: 18701448
- DOI: https://doi.org/10.1074/jbc.M804535200
- Primary Citation of Related Structures:
4LTD, 4LTM, 4LTN - PubMed Abstract:
EDTA has become a major organic pollutant in the environment because of its extreme usage and resistance to biodegradation. Recently, two critical enzymes, EDTA monooxygenase (EmoA) and NADH:FMN oxidoreductase (EmoB), belonging to the newly established two-component flavin-diffusible monooxygenase family, were identified in the EDTA degradation pathway in Mesorhizobium sp. BNC1. EmoA is an FMNH2-dependent enzyme that requires EmoB to provide FMNH2 for the conversion of EDTA to ethylenediaminediacetate. To understand the molecular basis of this FMN-mediated reaction, the crystal structures of the apo-form, FMN.FMN complex, and FMN.NADH complex of EmoB were determined at 2.5 angstroms resolution. The structure of EmoB is a homotetramer consisting of four alpha/beta-single-domain monomers of five parallel beta-strands flanked by five alpha-helices, which is quite different from those of other known two-component flavin-diffusible monooxygenase family members, such as PheA2 and HpaC, in terms of both tertiary and quaternary structures. For the first time, the crystal structures of both the FMN.FMN and FMN.NADH complexes of an NADH:FMN oxidoreductase were determined. Two stacked isoalloxazine rings and nicotinamide/isoalloxazine rings were at a proper distance for hydride transfer. The structures indicated a ping-pong reaction mechanism, which was confirmed by activity assays. Thus, the structural data offer detailed mechanistic information for hydride transfer between NADH to an enzyme-bound FMN and between the bound FMNH2 and a diffusible FMN.
- School of Molecular Biosciences, Washington State University, Pullman, Washington 99164-4660, USA.
Organizational Affiliation: