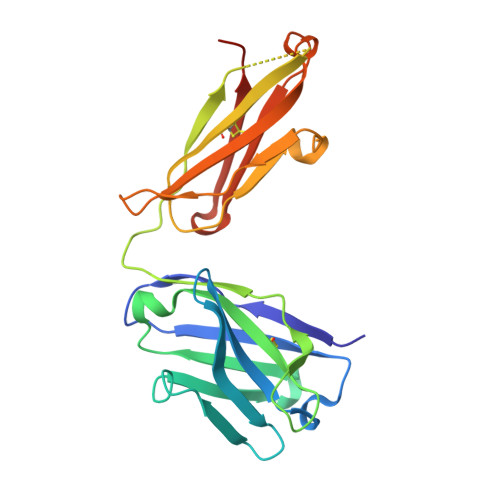
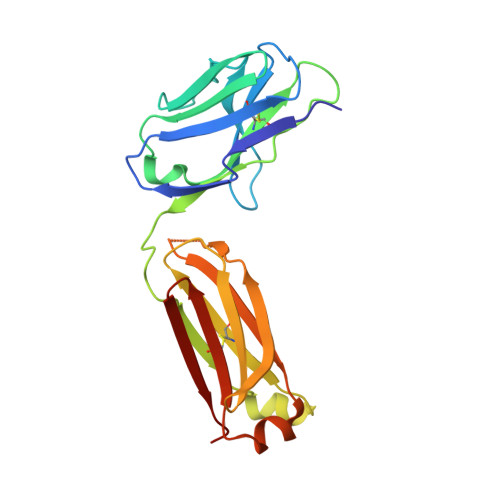
Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface.
Lewis, S.M., Wu, X., Pustilnik, A., Sereno, A., Huang, F., Rick, H.L., Guntas, G., Leaver-Fay, A., Smith, E.M., Ho, C., Hansen-Estruch, C., Chamberlain, A.K., Truhlar, S.M., Conner, E.M., Atwell, S., Kuhlman, B., Demarest, S.J.(2014) Nat Biotechnol 32: 191-198
- PubMed: 24463572
- DOI: https://doi.org/10.1038/nbt.2797
- Primary Citation of Related Structures:
4LLD, 4LLM, 4LLQ, 4LLU, 4LLW, 4LLY - PubMed Abstract:
Robust generation of IgG bispecific antibodies has been a long-standing challenge. Existing methods require extensive engineering of each individual antibody, discovery of common light chains, or complex and laborious biochemical processing. Here we combine computational and rational design approaches with experimental structural validation to generate antibody heavy and light chains with orthogonal Fab interfaces. Parental monoclonal antibodies incorporating these interfaces, when simultaneously co-expressed, assemble into bispecific IgG with improved heavy chain-light chain pairing. Bispecific IgGs generated with this approach exhibit pharmacokinetic and other desirable properties of native IgG, but bind target antigens monovalently. As such, these bispecific reagents may be useful in many biotechnological applications.
- 1] Department of Biochemistry and Biophysics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA. [2].
Organizational Affiliation: